Environmental Science
Pollution
AP Environmental Science
Water Pollution
Human Impacts on Ecosystem
Biodegradable Wastes
Great Pacific Garbage Patch
Persistent Organic Pollutants
Water Quality—Water Testing
Endocrine Disruptors
Wetlands
Mangroves
Bioaccumulation
Biomagnification
Solid Waste Disposal
Hazardous Wastes
12th

Chapter 8: Aquatic and Terrestrial Pollution
8.1: Sources of Water Pollution
Water pollution: It is the contamination of water bodies.
This form of environmental degradation occurs when pollutants are directly or indirectly discharged into water bodies without adequate treatment to remove harmful compounds.
Sources of Water Pollution
Point source pollution: Release pollutants from known locations, such as discharge pipes, that are regulated by federal and state agencies.
Non-point source pollution: A combination of pollutants from a large area rather than from specific identifiable sources
Thermal Pollution: The degradation of water quality by any process that changes ambient water temperature.
8.2: Human Impacts on Ecosystem
Cultural eutrophication: It is the process whereby human activity increases the amount of nutrients entering surface waters.
Ecological Effects of Cultural Eutrophication
Changes in species composition and dominance
Decomposed algae by anaerobic bacteria release toxic gases
Decreased biodiversity in water bodies
Dissolved oxygen (DO) depletion (hypoxia), resulting in fish kills
Increase in algal blooms
Increase in turbidity
Increased phytoplankton biomass
Toxic phytoplankton species
Human Activities That Contribute to Cultural Eutrophication
Discharge from water treatment facilities that do not have the capacity to handle nutrient and biodegradable waste discharge
Fertilizers and pesticides from residential and agricultural runoff
Sewer and drainage overflows can occur when the rainfall amount exceeds the wastewater treatment capacity
Use of household products that contain phosphates
Steps for Controlling Cultural Eutrophication
Controlling runoff from feedlots
Controlling the application and timing of applying fertilizer
Constructing wastewater lagoons and retention ponds near agricultural areas
Planting vegetation (buffer zones) along streambeds, which slows erosion and absorbs some of the excess nutrients
Updating building codes to utilize permeable pavement to absorb the excess urban runoff
Upgrading existing water treatment plants to better control nitrate and phosphate pollution through tertiary standards and other advanced technologies
Using monetary and tax incentives to convert existing watering systems to drip irrigation and to replace landscaping with native vegetation that is less water-demanding
Biodegradable Wastes
Nitrates: These are water-soluble and are found in fertilizers, which can remain on fields and accumulate, leach into groundwater, or end up in surface runoff and cause algal blooms in surface waters, resulting in decreased dissolved oxygen levels.
Phosphates: These are also a component of fertilizers; however, they are not water-soluble, and they adhere to soil particles.
Disease-causing microorganisms: Such as bacteria, viruses, and protozoa, can result in swimmers getting sick and shellfish becoming contaminated.
Mining
Cyanide is intentionally poured onto piles of mined rock to chemically extract gold.
Mining companies in developing nations dump waste into rivers and other waterways.
Mining releases earth-locked heavy metals and sulfur compounds.
Rainwater on tailings pollutes freshwater.
Effects of Oil Spills
Seabirds ingest their feather oil while preening, damaging their kidneys and livers.
Due to their limited foraging, they dehydrate quickly.
Oil floats on water, blocking sunlight from reaching marine plants and phytoplankton, affecting the ecosystem's food web.
Oil penetrates seabird feathers, making them less buoyant and more susceptible to temperature changes.
Dispersants, sorbents, and detergents disperse, absorb, or clump oil into sinking gel-like agglomerations.
Controlled burning, booming, skimming, and/or vacuuming oil from the surface or shoreline.
The use of microorganisms to break down the oil.
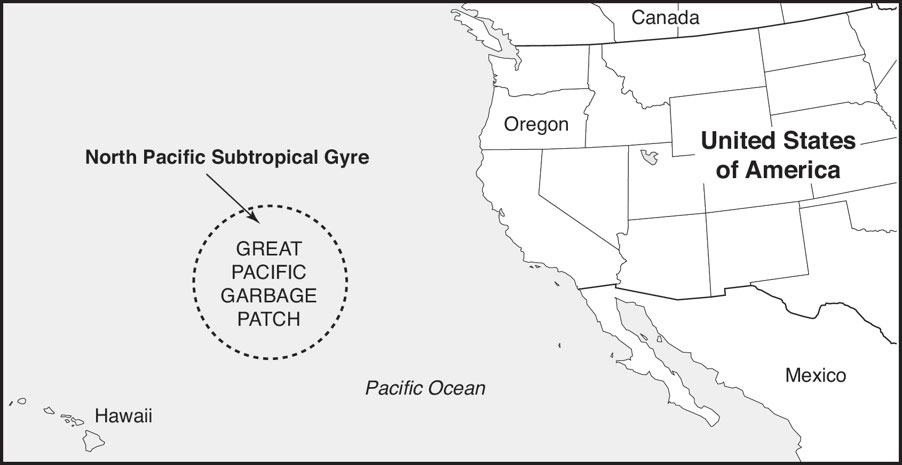
Great Pacific Garbage Patch
Great Pacific Garbage Patch: A large system of rotating ocean currents of marine litter in the central North Pacific Ocean and is characterized by high concentrations of floating plastics, chemical sludge, and other debris that have been trapped by the currents of the North Pacific Gyre.
Great Patch: Formed as a result of marine pollution gathered by oceanic currents as the gyre’s rotational pattern drew in waste material from across the North Pacific Ocean.
As materials are captured in the currents, the wind-driven surface currents gradually move floating debris toward and trap it in the center of the gyre.
As the plastic photodegrades into smaller and smaller pieces, it remains as plastic polymers leaching toxic chemicals into the upper water column.
As the plastic further disintegrates, it becomes small enough to be ingested by aquatic organisms and birds near the ocean’s surface and eventually enters the marine food chain.
8.3: Persistent Organic Pollutants (POPs)
Persistent Organic Pollutants: These are organic (carbon) compounds that are resistant to environmental degradation through chemical or biological processes or decomposition due to light.
It has been observed to persist in the environment, be capable of long-range transport, bioaccumulate in human and animal tissue, biomagnify in food chains, and have potentially significant impacts on human health and the environment.
Chemical characteristics of POPs include the following:
Ability to travel long distances through the atmosphere before being deposited on Earth.
A tendency to evaporate easily in hot regions and accumulate in cold regions, where they tend to condense and persist
High molecular masses
High-fat solubility, which makes them able to pass through biological membranes and bioaccumulate in the fatty tissues of living organisms
Low water solubility.
Urban Runoffs are the major source of urban flooding and water pollution in urban communities worldwide. It also creates:
create microclimates due to the high heat capacity of asphalt
fragment habitats;
increase groundwater depletion as water does not infiltrate into the soil to recharge aquifers; and
reduce biodiversity and seriously impact food webs in the area since there is less vegetation available for primary consumers.
Water Quality—Water Testing
Water quality: Refers to the chemical, physical, and biological characteristics of water and is a measure of the condition of the water relative to the requirements of one or more biotic species and/or to any human need or purpose.
Water testing: It is a broad description for various procedures that are used to analyze water quality.
Water Quality Tests
Alkalinity: It measures the sum of the bicarbonate, carbonate, and hydroxide ions in the water, which raise the pH.
The water source's ability to resist pH changes can increase fish egg, larva, and fry survival rates.
Ammonia: When found in natural water, is regarded as an indicator of pollution.
It is rapidly oxidized by certain bacteria in natural water systems into nitrite and nitrate.
Biological Oxygen Demand (BOD): It gives an approximation of the level of biodegradable waste in water.
Carbon Dioxide: Aquatic vegetation, ranging from phytoplankton to large rooted plants, depends upon carbon dioxide and bicarbonates in the water for growth.
When the oxygen concentration falls the carbon dioxide concentration increases and the pH increases.
High CO2 levels also make it difficult for fish to use the limited amount of oxygen present in the water and to discharge the CO2 in their bloodstream.
Low CO2 levels also result in a decreased rate of photosynthesis.
Dissolved Oxygen (DO): If its level is too low, it indicates possible water pollution and shows a potential for further pollution downstream because the ability of the stream to self-cleanse will be reduced.
Coliforms: These are a form of bacteria that are found in the intestines of warm-blooded animals; their presence in lakes, streams, and rivers is a sign of untreated sewage in the water.
Fecal coliforms: They can get into the water from untreated human sewage or from farms and runoff from animal feedlots.
Heavy Metals (Pb, Hg, Cd, Se)
As water becomes more acidic, metal solubility increases, and the metal particles become more mobile.
Metals can become “locked up” in bottom sediments, where they remain for many years.
Heavy metals are non-biodegradable and can cause decreased reproductive rates and birth defects.
Nitrate: This gets reduced to nitrites, which can be harmful to humans and fish.
Nitrite: Occurs in water as an intermediate product in the biological breakdown of organic nitrogen being produced either through the oxidation of ammonia or the reduction of nitrate.
pH
Changes in water pH can result in increased mortality of eggs and juveniles, decalcification of bone, and physiological stress.
Pollution tends to make water acidic and increases the solubility of heavy metals.
Most bodies of water have the highest biological diversity when the pH is near 7, with natural waters having pH values from 5.0 to 8.5.
Fresh rainwater may have a pH of 5.5 to 6.0 due to carbon dioxide dissolving in the water, making a weak carbonic acid solution.
Phosphates: These are essential nutrients for aquatic plants, but only in very low concentrations.
Excessive amounts of phosphorus build up easily, and small amounts can contaminate large volumes of water.
Salinity
Proper salinity levels are required to maintain osmotic pressure for living cells.
Decreased salinity also results in decreased DO and decreased viability of eggs and larvae.
Solids
A steady concentration of dissolved minerals is necessary to maintain the osmotic balance within the cells of organisms.
High levels of total dissolved solids (TDS) can affect water clarity and photosynthesis and lead to a decline in the quality and taste of drinking water.
Temperature
Higher water temperatures lower the amount of dissolved oxygen:
gases are less soluble in warmer water; and
warmer water increases the metabolic rate of aquatic organisms.
Higher temperatures also increase an organism’s sensitivity to toxic wastes and diseases.
Total hardness: It measures the total concentration of calcium and magnesium ions in the water.
Increased concentrations of these ions can increase the solubility of heavy metal ions in water and affect the water’s buffering capacity.
Turbidity: It is a measure of how light is scattered in the water column due to solids that do not dissolve but are small enough to be suspended in the water.
Drinking Water Treatment Methods
Absorption: When one substance enters completely into another.
Adsorption: When one substance just hangs onto the outside of another.
Disinfection: Using chemicals and/or cleansing techniques that destroy or prevent the growth of organisms that are capable of infection.
Filtration: Removes clays, natural organic matter, precipitants, and silts from the treatment process.
It clarifies water and enhances the effectiveness of disinfection.
Flocculation sedimentation: A process that combines small particles into larger particles that then settle out of the water as sediment
Ion exchange: Removes inorganic constituents and can be used to remove arsenic, chromium, excess fluoride, nitrates, radium, and uranium
8.4: Endocrine Disruptors
Gland: An organ that secretes particular chemical substances for use in the body or for discharge into the surroundings.
Endocrine System: A network of glands that make the hormones that help cells communicate with each other and is responsible for almost every cell, organ, and function in both humans and animals.
Endocrine disruptors: These are chemicals that can interfere with endocrine or hormonal systems and can cause behavior, learning and developmental disorders, birth defects, cancerous tumors, and loss of fertility.
Bisphenol A (BPA): Used in plastic manufacturing and epoxy.
Dioxins: By-product of herbicide production and paper bleaching, and released during burning wastes and wildfires.
Phthalates: Used to make plastics more flexible.
Polychlorinated biphenyls (PCBs): Used to make electrical equipment, heat transfer fluids and lubricants.
8.5: Human Impacts on Wetlands and Mangroves
Wetland: It is a place where the land is covered by water, which can be freshwater, saltwater, or brackish water.
It includes marshes, ponds, the edge of a lake or ocean, the delta at the mouth of a river, and low-lying areas that frequently flood.
Wetlands support high animal concentrations and serve as breeding grounds and nurseries for many species, making their destruction a major environmental issue.
Wetlands also allow for the cultivation of rice, a food source for half of the world’s population.
Mangrove: A shrub or small tree that grows in slightly salty (brackish) water formed by seawater mixing with freshwater in estuaries.
They have a complex salt filtration system and root system to survive salt water, low-oxygen mud, and wave action.
Since the Industrial Revolution, humans have had an increasingly negative impact on one of the most productive ecosystems on Earth through:
Diking and dredging;
Filling in these sensitive areas for urban development impacts water quality and runoff; and
An increase in hurricanes resulted in more frequent sea surges.
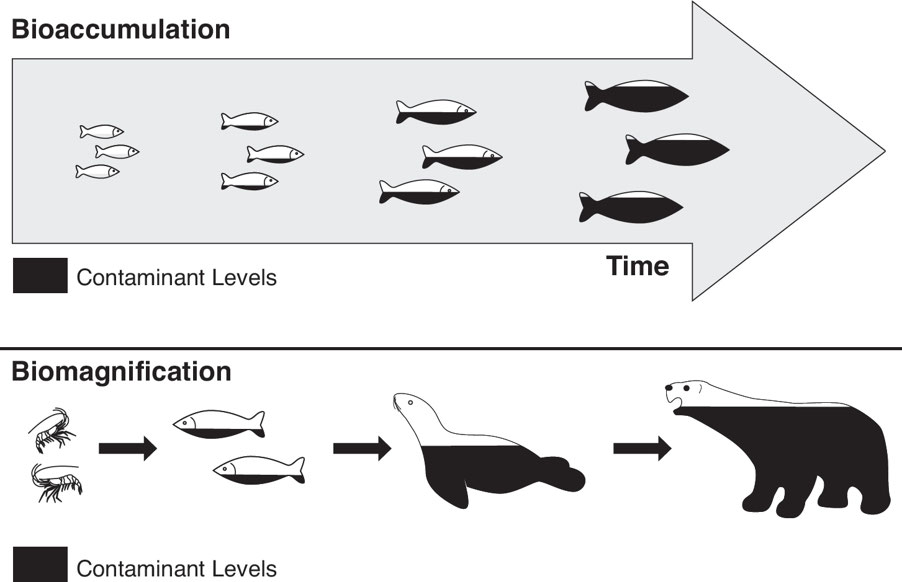
8.6: Bioaccumulation and Biomagnification
Bioaccumulation: It is the increase in the concentration of a pollutant within an organism.
The rate at which a given substance bioaccumulates depends upon the following:
The mode of uptake.
The degree of fat solubility of the pollutant
The rate at which the substance is eliminated from the organism
The transformation of the substance by metabolic processes
The lipid (fat) content of the organism
Biomagnification: It is the increasing concentration of a substance in the tissues of organisms at successively higher trophic levels within a food chain.
For biomagnification to occur, the following must be true:
The pollutant must be long-lived.
The pollutant must be mobile.
The pollutant must be soluble in fats.
The pollutant must be biologically active.
8.7: Solid Waste Disposal
Municipal solid waste (MSW): More commonly known as trash or garbage—consists of everyday items that are used and then thrown away.
Hazardous Wastes: Paints, chemicals, pesticides, etc.
These wastes can take hundreds of years to decompose.
Organic Wastes: Kitchen wastes, vegetables, flowers, leaves, or fruits.
Usually decompose within two weeks.
Wood can take 10 to 15 years to decompose.
Radioactive Wastes: Spent fuel rods and smoke detectors.
These can take hundreds of thousands of years to decompose.
Recyclable Wastes: Glass, metals, paper, and some plastics.
Paper decomposes in 10 to 30 days, while glass does not decompose.
Metals decompose in 100 to 500 years.
Some plastics can take up to 1 million years to decompose.
Soiled Wastes: Hospital wastes.
Cotton and cloth can take two to five months to decompose.
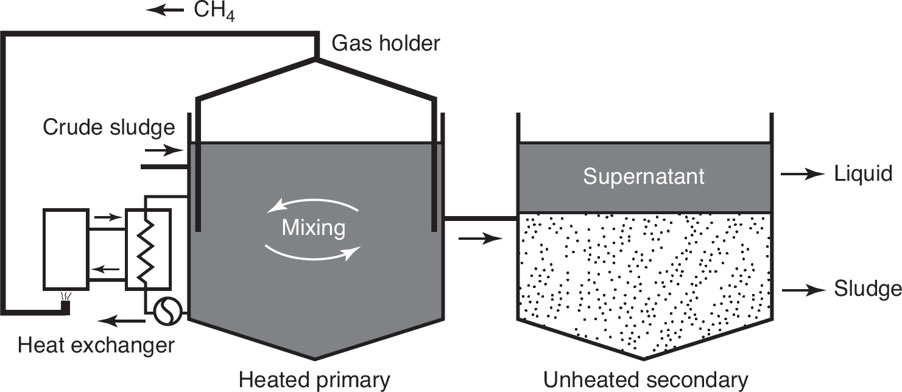
Anaerobic Digestion
Microorganisms: Are used to break down biodegradable material and sewage sludge in the absence of oxygen.
It is a renewable energy source.
It reduces the amount of organic matter, which might otherwise be destined to be dumped at sea or in landfills or burned in incinerators.
It reduces or eliminates the energy footprint of waste treatment plants.
It reduces the methane emission from landfills.
It is best suited for organic material and is commonly used for industrial effluent, wastewater, and sewage sludge treatment.
Nutrient-rich digestate can be used as fertilizer.
Waste Solutions and Energy Recovery
Incineration: A waste treatment process that involves the combustion of substances contained in waste materials and the conversion of the waste into ash, flue gas, and heat.
Global waste trade: It is the international trade of waste between countries for further treatment, disposal, and/or recycling.
Toxic or hazardous wastes are often exported from developed countries to developing countries.
Ocean dumping: The deliberate disposal of municipal and/or hazardous wastes at sea.
Sanitary landfills: Method of waste disposal where the waste is buried either underground or in large piles, and where waste is isolated from the environment until it is safe.
Reducing: Lessening the number of hazardous wastes by substituting and using products that are more “Earth-friendly.”
Freon®: Its molecular structure contains chlorine, which seriously degrades the stratospheric ozone layer.
Puron®: Substitutes fluorine for chlorine, and has less of an impact on the stratospheric ozone layer.
Hazardous Wastes
Radioactive wastes: Usually a by-product of nuclear power generation and other applications of nuclear fission, such as research and medicine.
This waste is hazardous to most forms of life and the environment and is regulated by government agencies to protect human health and the environment.
Low-level radioactive wastes: Contain low levels of radiation and remain dangerous for a relatively short time.
High-level radioactive wastes: Contain high levels of radiation and remain dangerous for a very long time
Reactive wastes: These are wastes that are unstable under normal conditions. Reactive wastes can cause explosions, gases, toxic fumes, or vapors when heated, compressed, or mixed with water.
Source-specific wastes: These are wastes from specific industries.
Teratogens: These are substances found in the environment that can cause birth defects.
Toxic wastes: These are wastes that are harmful or fatal when ingested or absorbed.
When disposed, these toxins may leach and pollute the groundwater.
Handling Hazardous Wastes
Landfill capping: A containment technology that forms a barrier between the contaminated media and the surface, protecting humans and the environment from its harmful effects and limiting its migration.
Hazardous waste caps consist of three layers:
An upper topsoil layer
A compacted soil barrier layer
A low-permeability layer made of a synthetic material
Hazardous waste landfills: Excavated or engineered sites for the final disposal of non-liquid hazardous waste are selected and designed to minimize environmental release.
Permanent storage: Isolates hazardous waste from the environment by condensing or concentrating it.
Methods Used to Isolate and Store Hazardous Wastes
Salt dome and bed formations, underground caves, and mines are geologic repositories.
Surface impoundments: These are natural topographic depressions, man-made excavations, or diked areas that are used for temporary storage and/or for the treatment of liquid hazardous waste.
Injection wells: These stores fluid deep underground in geologically stable, porous rock formations, such as sandstone or limestone, or into or below the shallow soil layer.
Waste piles: These are non-containerized piles of solid, non-liquid hazardous waste that are used for temporary storage or treatment.
Reduction and cleanup of hazardous wastes: These can occur by producing less waste, converting the hazardous material to less hazardous or nonhazardous substances, and placing the toxic material into perpetual storage.
Brownfield: It is land that was previously used for industrial or commercial purposes, may have been contaminated with hazardous wastes, and is commonly found in large urban areas.
Chapter 8: Aquatic and Terrestrial Pollution
8.1: Sources of Water Pollution
Water pollution: It is the contamination of water bodies.
This form of environmental degradation occurs when pollutants are directly or indirectly discharged into water bodies without adequate treatment to remove harmful compounds.
Sources of Water Pollution
Point source pollution: Release pollutants from known locations, such as discharge pipes, that are regulated by federal and state agencies.
Non-point source pollution: A combination of pollutants from a large area rather than from specific identifiable sources
Thermal Pollution: The degradation of water quality by any process that changes ambient water temperature.
8.2: Human Impacts on Ecosystem
Cultural eutrophication: It is the process whereby human activity increases the amount of nutrients entering surface waters.
Ecological Effects of Cultural Eutrophication
Changes in species composition and dominance
Decomposed algae by anaerobic bacteria release toxic gases
Decreased biodiversity in water bodies
Dissolved oxygen (DO) depletion (hypoxia), resulting in fish kills
Increase in algal blooms
Increase in turbidity
Increased phytoplankton biomass
Toxic phytoplankton species
Human Activities That Contribute to Cultural Eutrophication
Discharge from water treatment facilities that do not have the capacity to handle nutrient and biodegradable waste discharge
Fertilizers and pesticides from residential and agricultural runoff
Sewer and drainage overflows can occur when the rainfall amount exceeds the wastewater treatment capacity
Use of household products that contain phosphates
Steps for Controlling Cultural Eutrophication
Controlling runoff from feedlots
Controlling the application and timing of applying fertilizer
Constructing wastewater lagoons and retention ponds near agricultural areas
Planting vegetation (buffer zones) along streambeds, which slows erosion and absorbs some of the excess nutrients
Updating building codes to utilize permeable pavement to absorb the excess urban runoff
Upgrading existing water treatment plants to better control nitrate and phosphate pollution through tertiary standards and other advanced technologies
Using monetary and tax incentives to convert existing watering systems to drip irrigation and to replace landscaping with native vegetation that is less water-demanding
Biodegradable Wastes
Nitrates: These are water-soluble and are found in fertilizers, which can remain on fields and accumulate, leach into groundwater, or end up in surface runoff and cause algal blooms in surface waters, resulting in decreased dissolved oxygen levels.
Phosphates: These are also a component of fertilizers; however, they are not water-soluble, and they adhere to soil particles.
Disease-causing microorganisms: Such as bacteria, viruses, and protozoa, can result in swimmers getting sick and shellfish becoming contaminated.
Mining
Cyanide is intentionally poured onto piles of mined rock to chemically extract gold.
Mining companies in developing nations dump waste into rivers and other waterways.
Mining releases earth-locked heavy metals and sulfur compounds.
Rainwater on tailings pollutes freshwater.
Effects of Oil Spills
Seabirds ingest their feather oil while preening, damaging their kidneys and livers.
Due to their limited foraging, they dehydrate quickly.
Oil floats on water, blocking sunlight from reaching marine plants and phytoplankton, affecting the ecosystem's food web.
Oil penetrates seabird feathers, making them less buoyant and more susceptible to temperature changes.
Dispersants, sorbents, and detergents disperse, absorb, or clump oil into sinking gel-like agglomerations.
Controlled burning, booming, skimming, and/or vacuuming oil from the surface or shoreline.
The use of microorganisms to break down the oil.
Great Pacific Garbage Patch
Great Pacific Garbage Patch: A large system of rotating ocean currents of marine litter in the central North Pacific Ocean and is characterized by high concentrations of floating plastics, chemical sludge, and other debris that have been trapped by the currents of the North Pacific Gyre.
Great Patch: Formed as a result of marine pollution gathered by oceanic currents as the gyre’s rotational pattern drew in waste material from across the North Pacific Ocean.
As materials are captured in the currents, the wind-driven surface currents gradually move floating debris toward and trap it in the center of the gyre.
As the plastic photodegrades into smaller and smaller pieces, it remains as plastic polymers leaching toxic chemicals into the upper water column.
As the plastic further disintegrates, it becomes small enough to be ingested by aquatic organisms and birds near the ocean’s surface and eventually enters the marine food chain.

8.3: Persistent Organic Pollutants (POPs)
Persistent Organic Pollutants: These are organic (carbon) compounds that are resistant to environmental degradation through chemical or biological processes or decomposition due to light.
It has been observed to persist in the environment, be capable of long-range transport, bioaccumulate in human and animal tissue, biomagnify in food chains, and have potentially significant impacts on human health and the environment.
Chemical characteristics of POPs include the following:
Ability to travel long distances through the atmosphere before being deposited on Earth.
A tendency to evaporate easily in hot regions and accumulate in cold regions, where they tend to condense and persist
High molecular masses
High-fat solubility, which makes them able to pass through biological membranes and bioaccumulate in the fatty tissues of living organisms
Low water solubility.
Urban Runoffs are the major source of urban flooding and water pollution in urban communities worldwide. It also creates:
create microclimates due to the high heat capacity of asphalt
fragment habitats;
increase groundwater depletion as water does not infiltrate into the soil to recharge aquifers; and
reduce biodiversity and seriously impact food webs in the area since there is less vegetation available for primary consumers.
Water Quality—Water Testing
Water quality: Refers to the chemical, physical, and biological characteristics of water and is a measure of the condition of the water relative to the requirements of one or more biotic species and/or to any human need or purpose.
Water testing: It is a broad description for various procedures that are used to analyze water quality.
Water Quality Tests
Alkalinity: It measures the sum of the bicarbonate, carbonate, and hydroxide ions in the water, which raise the pH.
The water source's ability to resist pH changes can increase fish egg, larva, and fry survival rates.
Ammonia: When found in natural water, is regarded as an indicator of pollution.
It is rapidly oxidized by certain bacteria in natural water systems into nitrite and nitrate.
Biological Oxygen Demand (BOD): It gives an approximation of the level of biodegradable waste in water.
Carbon Dioxide: Aquatic vegetation, ranging from phytoplankton to large rooted plants, depends upon carbon dioxide and bicarbonates in the water for growth.
When the oxygen concentration falls the carbon dioxide concentration increases and the pH increases.
High CO2 levels also make it difficult for fish to use the limited amount of oxygen present in the water and to discharge the CO2 in their bloodstream.
Low CO2 levels also result in a decreased rate of photosynthesis.
Dissolved Oxygen (DO): If its level is too low, it indicates possible water pollution and shows a potential for further pollution downstream because the ability of the stream to self-cleanse will be reduced.
Coliforms: These are a form of bacteria that are found in the intestines of warm-blooded animals; their presence in lakes, streams, and rivers is a sign of untreated sewage in the water.
Fecal coliforms: They can get into the water from untreated human sewage or from farms and runoff from animal feedlots.
Heavy Metals (Pb, Hg, Cd, Se)
As water becomes more acidic, metal solubility increases, and the metal particles become more mobile.
Metals can become “locked up” in bottom sediments, where they remain for many years.
Heavy metals are non-biodegradable and can cause decreased reproductive rates and birth defects.
Nitrate: This gets reduced to nitrites, which can be harmful to humans and fish.
Nitrite: Occurs in water as an intermediate product in the biological breakdown of organic nitrogen being produced either through the oxidation of ammonia or the reduction of nitrate.
pH
Changes in water pH can result in increased mortality of eggs and juveniles, decalcification of bone, and physiological stress.
Pollution tends to make water acidic and increases the solubility of heavy metals.
Most bodies of water have the highest biological diversity when the pH is near 7, with natural waters having pH values from 5.0 to 8.5.
Fresh rainwater may have a pH of 5.5 to 6.0 due to carbon dioxide dissolving in the water, making a weak carbonic acid solution.
Phosphates: These are essential nutrients for aquatic plants, but only in very low concentrations.
Excessive amounts of phosphorus build up easily, and small amounts can contaminate large volumes of water.
Salinity
Proper salinity levels are required to maintain osmotic pressure for living cells.
Decreased salinity also results in decreased DO and decreased viability of eggs and larvae.
Solids
A steady concentration of dissolved minerals is necessary to maintain the osmotic balance within the cells of organisms.
High levels of total dissolved solids (TDS) can affect water clarity and photosynthesis and lead to a decline in the quality and taste of drinking water.
Temperature
Higher water temperatures lower the amount of dissolved oxygen:
gases are less soluble in warmer water; and
warmer water increases the metabolic rate of aquatic organisms.
Higher temperatures also increase an organism’s sensitivity to toxic wastes and diseases.
Total hardness: It measures the total concentration of calcium and magnesium ions in the water.
Increased concentrations of these ions can increase the solubility of heavy metal ions in water and affect the water’s buffering capacity.
Turbidity: It is a measure of how light is scattered in the water column due to solids that do not dissolve but are small enough to be suspended in the water.
Drinking Water Treatment Methods
Absorption: When one substance enters completely into another.
Adsorption: When one substance just hangs onto the outside of another.
Disinfection: Using chemicals and/or cleansing techniques that destroy or prevent the growth of organisms that are capable of infection.
Filtration: Removes clays, natural organic matter, precipitants, and silts from the treatment process.
It clarifies water and enhances the effectiveness of disinfection.
Flocculation sedimentation: A process that combines small particles into larger particles that then settle out of the water as sediment
Ion exchange: Removes inorganic constituents and can be used to remove arsenic, chromium, excess fluoride, nitrates, radium, and uranium
8.4: Endocrine Disruptors
Gland: An organ that secretes particular chemical substances for use in the body or for discharge into the surroundings.
Endocrine System: A network of glands that make the hormones that help cells communicate with each other and is responsible for almost every cell, organ, and function in both humans and animals.
Endocrine disruptors: These are chemicals that can interfere with endocrine or hormonal systems and can cause behavior, learning and developmental disorders, birth defects, cancerous tumors, and loss of fertility.
Bisphenol A (BPA): Used in plastic manufacturing and epoxy.
Dioxins: By-product of herbicide production and paper bleaching, and released during burning wastes and wildfires.
Phthalates: Used to make plastics more flexible.
Polychlorinated biphenyls (PCBs): Used to make electrical equipment, heat transfer fluids and lubricants.
8.5: Human Impacts on Wetlands and Mangroves
Wetland: It is a place where the land is covered by water, which can be freshwater, saltwater, or brackish water.
It includes marshes, ponds, the edge of a lake or ocean, the delta at the mouth of a river, and low-lying areas that frequently flood.
Wetlands support high animal concentrations and serve as breeding grounds and nurseries for many species, making their destruction a major environmental issue.
Wetlands also allow for the cultivation of rice, a food source for half of the world’s population.
Mangrove: A shrub or small tree that grows in slightly salty (brackish) water formed by seawater mixing with freshwater in estuaries.
They have a complex salt filtration system and root system to survive salt water, low-oxygen mud, and wave action.
Since the Industrial Revolution, humans have had an increasingly negative impact on one of the most productive ecosystems on Earth through:
Diking and dredging;
Filling in these sensitive areas for urban development impacts water quality and runoff; and
An increase in hurricanes resulted in more frequent sea surges.
8.6: Bioaccumulation and Biomagnification
Bioaccumulation: It is the increase in the concentration of a pollutant within an organism.
The rate at which a given substance bioaccumulates depends upon the following:
The mode of uptake.
The degree of fat solubility of the pollutant
The rate at which the substance is eliminated from the organism
The transformation of the substance by metabolic processes
The lipid (fat) content of the organism
Biomagnification: It is the increasing concentration of a substance in the tissues of organisms at successively higher trophic levels within a food chain.
For biomagnification to occur, the following must be true:
The pollutant must be long-lived.
The pollutant must be mobile.
The pollutant must be soluble in fats.
The pollutant must be biologically active.

8.7: Solid Waste Disposal
Municipal solid waste (MSW): More commonly known as trash or garbage—consists of everyday items that are used and then thrown away.
Hazardous Wastes: Paints, chemicals, pesticides, etc.
These wastes can take hundreds of years to decompose.
Organic Wastes: Kitchen wastes, vegetables, flowers, leaves, or fruits.
Usually decompose within two weeks.
Wood can take 10 to 15 years to decompose.
Radioactive Wastes: Spent fuel rods and smoke detectors.
These can take hundreds of thousands of years to decompose.
Recyclable Wastes: Glass, metals, paper, and some plastics.
Paper decomposes in 10 to 30 days, while glass does not decompose.
Metals decompose in 100 to 500 years.
Some plastics can take up to 1 million years to decompose.
Soiled Wastes: Hospital wastes.
Cotton and cloth can take two to five months to decompose.
Anaerobic Digestion
Microorganisms: Are used to break down biodegradable material and sewage sludge in the absence of oxygen.
It is a renewable energy source.
It reduces the amount of organic matter, which might otherwise be destined to be dumped at sea or in landfills or burned in incinerators.
It reduces or eliminates the energy footprint of waste treatment plants.
It reduces the methane emission from landfills.
It is best suited for organic material and is commonly used for industrial effluent, wastewater, and sewage sludge treatment.
Nutrient-rich digestate can be used as fertilizer.

Waste Solutions and Energy Recovery
Incineration: A waste treatment process that involves the combustion of substances contained in waste materials and the conversion of the waste into ash, flue gas, and heat.
Global waste trade: It is the international trade of waste between countries for further treatment, disposal, and/or recycling.
Toxic or hazardous wastes are often exported from developed countries to developing countries.
Ocean dumping: The deliberate disposal of municipal and/or hazardous wastes at sea.
Sanitary landfills: Method of waste disposal where the waste is buried either underground or in large piles, and where waste is isolated from the environment until it is safe.
Reducing: Lessening the number of hazardous wastes by substituting and using products that are more “Earth-friendly.”
Freon®: Its molecular structure contains chlorine, which seriously degrades the stratospheric ozone layer.
Puron®: Substitutes fluorine for chlorine, and has less of an impact on the stratospheric ozone layer.
Hazardous Wastes
Radioactive wastes: Usually a by-product of nuclear power generation and other applications of nuclear fission, such as research and medicine.
This waste is hazardous to most forms of life and the environment and is regulated by government agencies to protect human health and the environment.
Low-level radioactive wastes: Contain low levels of radiation and remain dangerous for a relatively short time.
High-level radioactive wastes: Contain high levels of radiation and remain dangerous for a very long time
Reactive wastes: These are wastes that are unstable under normal conditions. Reactive wastes can cause explosions, gases, toxic fumes, or vapors when heated, compressed, or mixed with water.
Source-specific wastes: These are wastes from specific industries.
Teratogens: These are substances found in the environment that can cause birth defects.
Toxic wastes: These are wastes that are harmful or fatal when ingested or absorbed.
When disposed, these toxins may leach and pollute the groundwater.
Handling Hazardous Wastes
Landfill capping: A containment technology that forms a barrier between the contaminated media and the surface, protecting humans and the environment from its harmful effects and limiting its migration.
Hazardous waste caps consist of three layers:
An upper topsoil layer
A compacted soil barrier layer
A low-permeability layer made of a synthetic material
Hazardous waste landfills: Excavated or engineered sites for the final disposal of non-liquid hazardous waste are selected and designed to minimize environmental release.
Permanent storage: Isolates hazardous waste from the environment by condensing or concentrating it.
Methods Used to Isolate and Store Hazardous Wastes
Salt dome and bed formations, underground caves, and mines are geologic repositories.
Surface impoundments: These are natural topographic depressions, man-made excavations, or diked areas that are used for temporary storage and/or for the treatment of liquid hazardous waste.
Injection wells: These stores fluid deep underground in geologically stable, porous rock formations, such as sandstone or limestone, or into or below the shallow soil layer.
Waste piles: These are non-containerized piles of solid, non-liquid hazardous waste that are used for temporary storage or treatment.
Reduction and cleanup of hazardous wastes: These can occur by producing less waste, converting the hazardous material to less hazardous or nonhazardous substances, and placing the toxic material into perpetual storage.
Brownfield: It is land that was previously used for industrial or commercial purposes, may have been contaminated with hazardous wastes, and is commonly found in large urban areas.
 Knowt
Knowt