
Chapter 1- Kinetic Particle Theory
Matter is a substance that has a mass and occupies space.
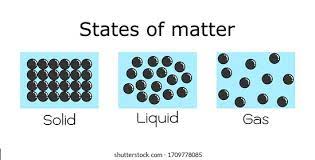
There are three main types of matter: Solid, liquid and gases.
Characteristic | Solid | Liquid | Gases |
|---|---|---|---|
Arrangement of particles | Orderly and closely packed | Disorderly and less closely packed than a solid | Disorderly and very far apart |
Attractive forces between particles | Very strong | Strong | Weak |
Kinetic energy of particles | Very low | Low | High |
Motion of particles | Vibrate and rotate about fixed positions | Move freely throughout the liquid | Move about rapidly in all directions |
Shape | Fixed | Not fixed | Not fixed |
Volume | Fixed | Fixed | Fixed |
Compressibility | Cannot be compressed | Cannot be compressed | Can be compressed |
WHY DO SOLIDS HAVE A FIXED SHAPE?
Due to strong forces of attraction between its particles, they are held tightly together and cannot move out freely. They also have just enough kinetic energy to vibrate in their fixed positions.
WHY DO SOLIDS HAVE A FIXED VOLUME?
The particles of a solid are already very close to one another.
WHY DOES A LIQUID NOT HAVE A FIXED SHAPE?
Forces of attraction between liquid molecules are weaker than those in solids. Therefore, the molecules can slide over one another and a liquid does not have a fixed shape.
WHY DOES A LIQUID HAVE A FIXED VOLUME?
Although liquid molecules are farther apart from eachother than solid molecules, they are still pretty close so liquids cannot be compressed.
WHY DOES A GAS HAVE AN UNFIXED SHAPE AND VOLUME?
The particles in a gas are very far apart with very weak forces of attraction and high kinetic energies. Since a gas can be compressed, it does not have a fixed volume.
KINETIC PARTICLE THEORY
The Kinetic Particle Theory states that all matter is made up of tiny particles that are in constant and random motion.
It also:
describes the states of matter
explains the difference in properties of solids, liquids and gases.
explains changes of state.
Different states of matter have different kinetic energies. Due to changes in the surrounding temperatures, matter may gain or lose energy resulting in reversible changes called state change.
States of matter can be changed from one form to another. Different processes include:
Freezing: a substance changes from a liquid to a solid at a fixed temperature called the freezing point. Energy is given out.
STEPS:
Heat energy is given out by particles in liquid.
Kinetic energy is lost and the particles start to move slower about their fixed positions.
When the energy becomes low enough, particles start to settle in fixed positions. There is no temperature change during this stage.
The particles completely settle and can only vibrate about their fixed positions. The liquid becomes a solid.
Melting: a substance changes from a solid to a liquid at a fixed temperature called the melting point. Energy is taken in.
STEPS:
Heat energy is absorbed by particles in the solid.
Heat energy is converted to kinetic energy and the particles start to move faster about their fixed positions.
When the energy becomes sufficient, particles overcome their forces of attraction and begin to break away. There is no temperature change during this stage.
The particles completely overcome their forces of attraction and the solid becomes a liquid.
Boiling: a substance changes from a liquid to a gas at a fixed temperature called the boiling point. Energy Is taken in.
Evaporation: a substance changes from a solid to a liquid at temperatures lower than its boiling point. Energy is taken in.
Condensation: a substance changes from a solid to a liquid.
Sublimation: Solids change directly to gases (or vice versa) without first becoming a liquid. It occurs when solid particles have sufficient energy to directly change into the gas.
HOW IS SUBLIMATION USEFUL?
For insecticide medicines that directly change to gas, spreading fumes.
For industrial refrigeration, dry ice is used so it doesn’t leave any liquid behind.
Differences between boiling and evaporation
Boiling | Evaporation |
|---|---|
Occurs at a boiling point only. | Occurs at a range of temperatures below the boiling point. |
Occurs throughout the liquid. | Occurs only at the surface. |
Rapid. | Slow. |
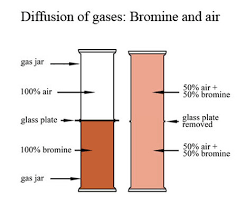
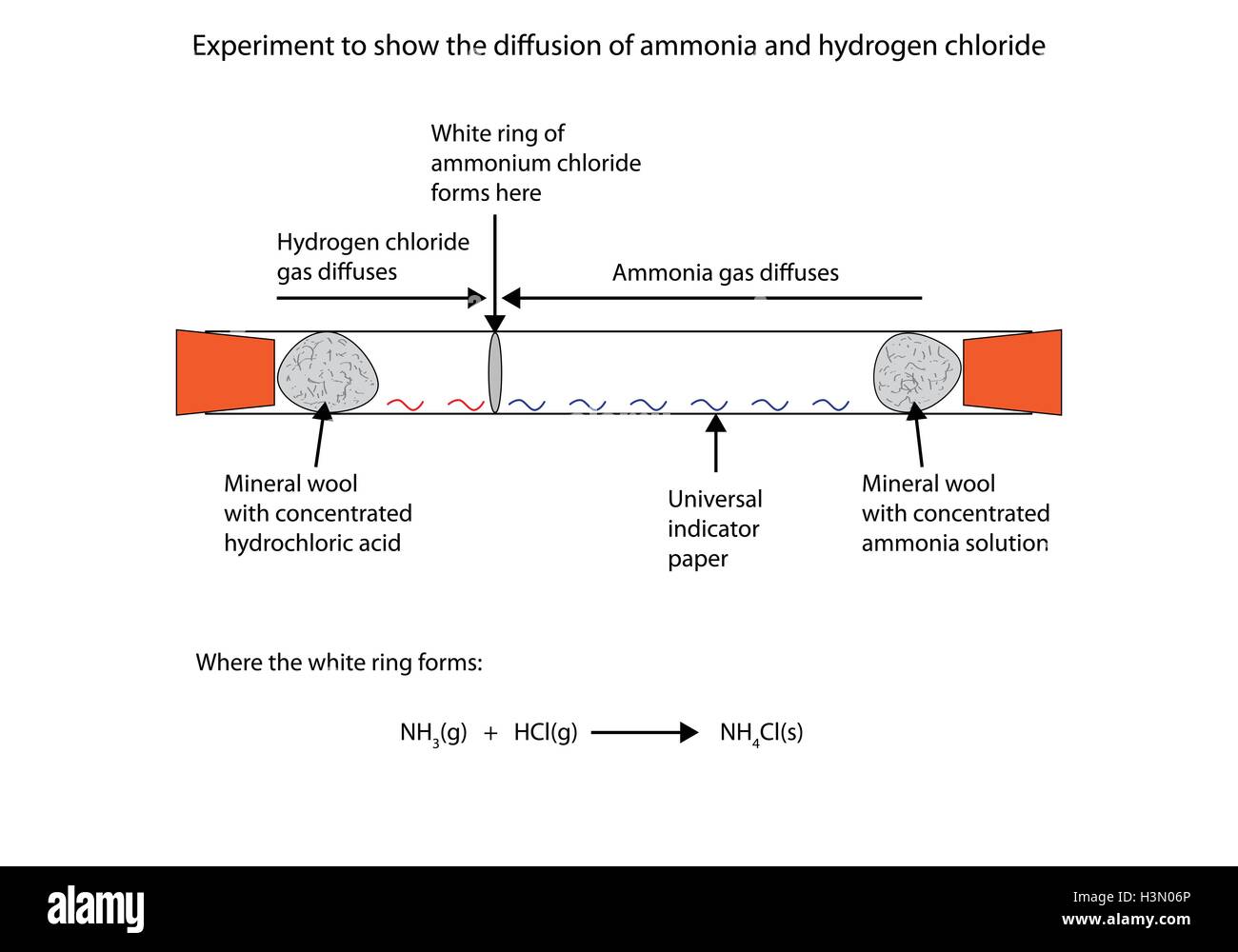
DIFFUSION
Diffusion: The movement of particles from higher to lower concentrations to fill up any space is diffusion.
It depends on the molecular mass (rate of diffusion decreases with an increase in mass) and temperature (rate of diffusion increases with an increase in temperature.)
Chapter 1- Kinetic Particle Theory
Matter is a substance that has a mass and occupies space.
There are three main types of matter: Solid, liquid and gases.
Characteristic | Solid | Liquid | Gases |
|---|---|---|---|
Arrangement of particles | Orderly and closely packed | Disorderly and less closely packed than a solid | Disorderly and very far apart |
Attractive forces between particles | Very strong | Strong | Weak |
Kinetic energy of particles | Very low | Low | High |
Motion of particles | Vibrate and rotate about fixed positions | Move freely throughout the liquid | Move about rapidly in all directions |
Shape | Fixed | Not fixed | Not fixed |
Volume | Fixed | Fixed | Fixed |
Compressibility | Cannot be compressed | Cannot be compressed | Can be compressed |

WHY DO SOLIDS HAVE A FIXED SHAPE?
Due to strong forces of attraction between its particles, they are held tightly together and cannot move out freely. They also have just enough kinetic energy to vibrate in their fixed positions.
WHY DO SOLIDS HAVE A FIXED VOLUME?
The particles of a solid are already very close to one another.
WHY DOES A LIQUID NOT HAVE A FIXED SHAPE?
Forces of attraction between liquid molecules are weaker than those in solids. Therefore, the molecules can slide over one another and a liquid does not have a fixed shape.
WHY DOES A LIQUID HAVE A FIXED VOLUME?
Although liquid molecules are farther apart from eachother than solid molecules, they are still pretty close so liquids cannot be compressed.
WHY DOES A GAS HAVE AN UNFIXED SHAPE AND VOLUME?
The particles in a gas are very far apart with very weak forces of attraction and high kinetic energies. Since a gas can be compressed, it does not have a fixed volume.
KINETIC PARTICLE THEORY
The Kinetic Particle Theory states that all matter is made up of tiny particles that are in constant and random motion.
It also:
describes the states of matter
explains the difference in properties of solids, liquids and gases.
explains changes of state.
Different states of matter have different kinetic energies. Due to changes in the surrounding temperatures, matter may gain or lose energy resulting in reversible changes called state change.
States of matter can be changed from one form to another. Different processes include:
Freezing: a substance changes from a liquid to a solid at a fixed temperature called the freezing point. Energy is given out.
STEPS:
Heat energy is given out by particles in liquid.
Kinetic energy is lost and the particles start to move slower about their fixed positions.
When the energy becomes low enough, particles start to settle in fixed positions. There is no temperature change during this stage.
The particles completely settle and can only vibrate about their fixed positions. The liquid becomes a solid.
Melting: a substance changes from a solid to a liquid at a fixed temperature called the melting point. Energy is taken in.
STEPS:
Heat energy is absorbed by particles in the solid.
Heat energy is converted to kinetic energy and the particles start to move faster about their fixed positions.
When the energy becomes sufficient, particles overcome their forces of attraction and begin to break away. There is no temperature change during this stage.
The particles completely overcome their forces of attraction and the solid becomes a liquid.
Boiling: a substance changes from a liquid to a gas at a fixed temperature called the boiling point. Energy Is taken in.
Evaporation: a substance changes from a solid to a liquid at temperatures lower than its boiling point. Energy is taken in.
Condensation: a substance changes from a solid to a liquid.
Sublimation: Solids change directly to gases (or vice versa) without first becoming a liquid. It occurs when solid particles have sufficient energy to directly change into the gas.
HOW IS SUBLIMATION USEFUL?
For insecticide medicines that directly change to gas, spreading fumes.
For industrial refrigeration, dry ice is used so it doesn’t leave any liquid behind.
Differences between boiling and evaporation
Boiling | Evaporation |
|---|---|
Occurs at a boiling point only. | Occurs at a range of temperatures below the boiling point. |
Occurs throughout the liquid. | Occurs only at the surface. |
Rapid. | Slow. |
DIFFUSION
Diffusion: The movement of particles from higher to lower concentrations to fill up any space is diffusion.
It depends on the molecular mass (rate of diffusion decreases with an increase in mass) and temperature (rate of diffusion increases with an increase in temperature.)


 Knowt
Knowt
