
Chapter 4: Atoms and Elements
4.1: Elements and Symbols
Elements
These are pure substances from which all other things are built.
These cannot be broken down into simpler substances.
Chemical Symbols
These are one- or two-letter abbreviations for the names of the elements.
Only the first letter of an element’s symbol is capitalized.
If the symbol has a second letter, it is lowercase so that we know when a different element is indicated.
If two letters are capitalized, they represent the symbols of two different elements.
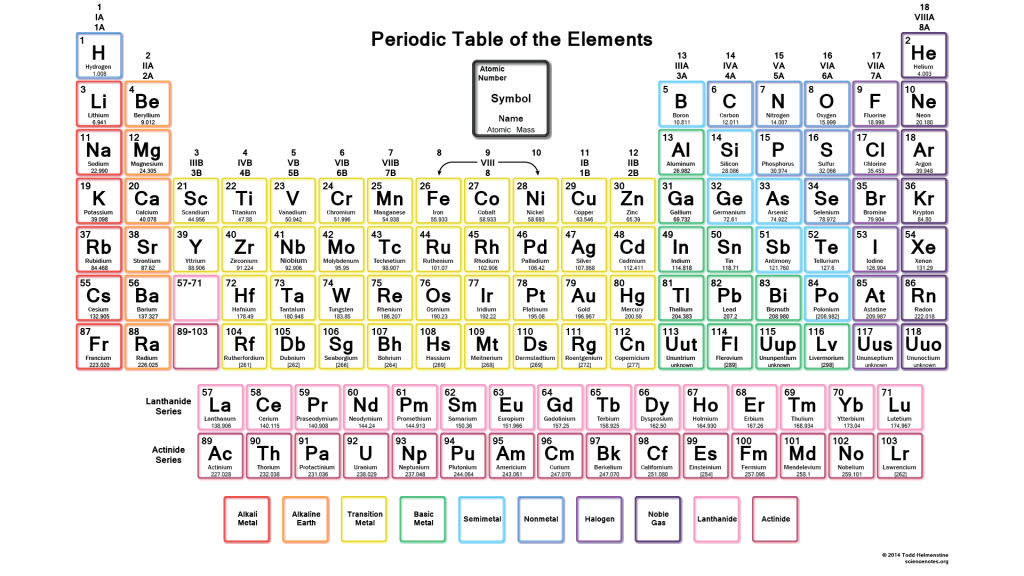
4.2: The Periodic Table
Periodic Table: The arrangement of 118 elements.
Period: Each horizontal row in the periodic table.
Group: Each vertical column on the periodic table.
Representative Elements: These have group numbers 1A to 8A.
Transition Elements: These are block elements in the center of a periodic table that have group numbers followed by the letter ‘B’.
The two rows of 14 elements called the lanthanides and actinides, which are part of Periods 6 and 7, are placed at the bottom of the periodic table to allow them to fit on a page.
Group 1A: These are alkali metals except for Hydrogen.
Group 2A: These are alkaline earth metals; these are shiny metals but not as reactive.
Group 7A: These are halogens; highly reactive and form compounds with most of the elements.
Group 8A: These are noble gases; they are quite unreactive and are seldom found in combination with other elements.
Metals: These are shiny solids, such as copper, gold, and silver. It can be shaped into wires or hammered into a flat sheet.
They are good conductors of heat and electricity. They usually heat at a higher temperature than nonmetals.
Nonmetals: They are often poor conductors of heat and electricity.
They have low melting points and low densities.
Metalloids: These are elements that exhibit some properties that are typical of metals and other properties that are characteristic of nonmetals.
These are semiconductors because they can be modified to function as conductors or insulators.
4.3: The Atom
Atom: The smallest unit particle of an element that retains the characteristics of that element.
Subatomic Particles: These are even smaller bits of matter.
Cathode Rays: These are streams of small particles produced when electricity is applied to a glass tube.
Protons: These are the atom’s positively charged particles.
Electrons: These are the atom’s negatively charged particles.
Neutrons: These are the atom’s neutral particles.
Nucleus: The center of the atom.
Atomic Mass Unit: A very small unit of mass.
It is defined as one-twelfth of the mass of a carbon atom which has a nucleus containing 6 protons and 6 neutrons.
Dalton: The atomic mass unit.
Dalton’s Atomic Theory
All matter is made up of tiny particles called atoms.
All atoms of a given element are similar to one another and different from atoms of other elements.
Atoms of two or more different elements combine to form compounds. A particular compound is always made up of the same kinds of atoms and always has the same number of each kind of atom.
A chemical reaction involves the rearrangement, separation, or combination of atoms. Atoms are never created or destroyed during a chemical reaction.
4.4: Atomic Number and Mass Number
Atomic Number: This is equal to the number of protons in every atom of that element.
It is the whole number that appears above the symbol of each element on the periodic table.
Atomic number = number of protons in an atom
Mass Number: The total number of protons and neutrons in its nucleus.
It does not appear on the periodic table because it applies to single atoms only.
Mass number = number of protons + number of neutrons
If we are given the mass number of an atom and its atomic number, we can calculate the number of neutrons in its nucleus.
Number of neutrons in a nucleus = mass number - number of protons
4.5: Isotopes and Atomic Mass
Isotopes: These are atoms of the same element that have the same atomic number but different numbers of neutrons.
Atomic Symbol: It indicates the mass number in the upper left corner and the atomic number in the lower left corner.
Because each isotope has a different mass, chemists have calculated an atomic mass for an “average atom,” which is a weighted average of the masses of all the naturally occurring isotopes of that element.
4.6: Electron and Energy Levels
Electromagnetic spectrum: It shows the arrangement of different types of electromagnetic radiation in order of increasing energy.
When the light from the Sun passes through a prism, the light separates into a continuous color spectrum, which consists of the colors we see in a rainbow.
When light from a heated element passes through a prism, it separates into distinct lines of color separated by dark areas called an atomic spectrum.
In an atom, each electron has a specific energy known as its energy level, which is assigned values called principal quantum numbers.
An electron can change from one energy level to a higher level only if it absorbs the energy equal to the difference in energy levels.
When an electron changes to a lower energy level, it emits energy equal to the difference between the two levels.
If the energy emitted is in the visible range, we see one of the colors of visible light.
The electron arrangement of an atom gives the number of electrons in each energy level.
4.7: Trends in Periodic Properties
Period Properties: These consists of the valence electrons in atoms, the trends in atomic size, ionization energy, and metallic character.
Valence Electrons: These are the electrons in the outermost energy level.
Electron-Dot Symbol: Also known as a Lewis Structure, represents the valence electrons as dots that are placed on the sides, top, or bottom of the symbol for the element.
Ionization Energy: A quantity of energy that is required to remove one of the outermost electrons.
Cation: A positive particle.
Anion: A negative particle.
The ionization energy decreases going down a group.
Going across a period from left to right, the ionization energy increases.
Metallic Character: An element that loses valence electrons easily.
It is more prevalent in the elements on the left side of the periodic table and decreases going from left to right across a period.
The elements on the right side of the periodic table do not easily lose electrons, which means they are the least metallic.
Atoms at the bottom of any group have more electron levels, which makes it easier to lose electrons
Chapter 4: Atoms and Elements
4.1: Elements and Symbols
Elements
These are pure substances from which all other things are built.
These cannot be broken down into simpler substances.
Chemical Symbols
These are one- or two-letter abbreviations for the names of the elements.
Only the first letter of an element’s symbol is capitalized.
If the symbol has a second letter, it is lowercase so that we know when a different element is indicated.
If two letters are capitalized, they represent the symbols of two different elements.
4.2: The Periodic Table
Periodic Table: The arrangement of 118 elements.
Period: Each horizontal row in the periodic table.
Group: Each vertical column on the periodic table.
Representative Elements: These have group numbers 1A to 8A.
Transition Elements: These are block elements in the center of a periodic table that have group numbers followed by the letter ‘B’.
The two rows of 14 elements called the lanthanides and actinides, which are part of Periods 6 and 7, are placed at the bottom of the periodic table to allow them to fit on a page.
Group 1A: These are alkali metals except for Hydrogen.
Group 2A: These are alkaline earth metals; these are shiny metals but not as reactive.
Group 7A: These are halogens; highly reactive and form compounds with most of the elements.
Group 8A: These are noble gases; they are quite unreactive and are seldom found in combination with other elements.
Metals: These are shiny solids, such as copper, gold, and silver. It can be shaped into wires or hammered into a flat sheet.
They are good conductors of heat and electricity. They usually heat at a higher temperature than nonmetals.
Nonmetals: They are often poor conductors of heat and electricity.
They have low melting points and low densities.
Metalloids: These are elements that exhibit some properties that are typical of metals and other properties that are characteristic of nonmetals.
These are semiconductors because they can be modified to function as conductors or insulators.

4.3: The Atom
Atom: The smallest unit particle of an element that retains the characteristics of that element.
Subatomic Particles: These are even smaller bits of matter.
Cathode Rays: These are streams of small particles produced when electricity is applied to a glass tube.
Protons: These are the atom’s positively charged particles.
Electrons: These are the atom’s negatively charged particles.
Neutrons: These are the atom’s neutral particles.
Nucleus: The center of the atom.
Atomic Mass Unit: A very small unit of mass.
It is defined as one-twelfth of the mass of a carbon atom which has a nucleus containing 6 protons and 6 neutrons.
Dalton: The atomic mass unit.
Dalton’s Atomic Theory
All matter is made up of tiny particles called atoms.
All atoms of a given element are similar to one another and different from atoms of other elements.
Atoms of two or more different elements combine to form compounds. A particular compound is always made up of the same kinds of atoms and always has the same number of each kind of atom.
A chemical reaction involves the rearrangement, separation, or combination of atoms. Atoms are never created or destroyed during a chemical reaction.
4.4: Atomic Number and Mass Number
Atomic Number: This is equal to the number of protons in every atom of that element.
It is the whole number that appears above the symbol of each element on the periodic table.
Atomic number = number of protons in an atom
Mass Number: The total number of protons and neutrons in its nucleus.
It does not appear on the periodic table because it applies to single atoms only.
Mass number = number of protons + number of neutrons
If we are given the mass number of an atom and its atomic number, we can calculate the number of neutrons in its nucleus.
Number of neutrons in a nucleus = mass number - number of protons
4.5: Isotopes and Atomic Mass
Isotopes: These are atoms of the same element that have the same atomic number but different numbers of neutrons.
Atomic Symbol: It indicates the mass number in the upper left corner and the atomic number in the lower left corner.
Because each isotope has a different mass, chemists have calculated an atomic mass for an “average atom,” which is a weighted average of the masses of all the naturally occurring isotopes of that element.
4.6: Electron and Energy Levels
Electromagnetic spectrum: It shows the arrangement of different types of electromagnetic radiation in order of increasing energy.
When the light from the Sun passes through a prism, the light separates into a continuous color spectrum, which consists of the colors we see in a rainbow.
When light from a heated element passes through a prism, it separates into distinct lines of color separated by dark areas called an atomic spectrum.
In an atom, each electron has a specific energy known as its energy level, which is assigned values called principal quantum numbers.
An electron can change from one energy level to a higher level only if it absorbs the energy equal to the difference in energy levels.
When an electron changes to a lower energy level, it emits energy equal to the difference between the two levels.
If the energy emitted is in the visible range, we see one of the colors of visible light.
The electron arrangement of an atom gives the number of electrons in each energy level.
4.7: Trends in Periodic Properties
Period Properties: These consists of the valence electrons in atoms, the trends in atomic size, ionization energy, and metallic character.
Valence Electrons: These are the electrons in the outermost energy level.
Electron-Dot Symbol: Also known as a Lewis Structure, represents the valence electrons as dots that are placed on the sides, top, or bottom of the symbol for the element.
Ionization Energy: A quantity of energy that is required to remove one of the outermost electrons.
Cation: A positive particle.
Anion: A negative particle.
The ionization energy decreases going down a group.
Going across a period from left to right, the ionization energy increases.
Metallic Character: An element that loses valence electrons easily.
It is more prevalent in the elements on the left side of the periodic table and decreases going from left to right across a period.
The elements on the right side of the periodic table do not easily lose electrons, which means they are the least metallic.
Atoms at the bottom of any group have more electron levels, which makes it easier to lose electrons
 Knowt
Knowt
