Lab Report: Deicer
Finding out if NaCl is the best Deicer for Minnesota Roads by Increasing Water's Freezing Point and Observing Dissolution Specific heat
Riley Mills, Angel Doss, Emily Eydel and Matthew Hang
February 28th, Spring 2023
Abstract
In this experiment a variety of tests were performed to find out if Sodium chloride (NaCl) was a good deicer for Minnesota roads. The test that were performed were finding the molarity (x axis) versus the change in freezing point (y axis)and finding the enthalpy of dissolution for NaCl. The graph has a slope that is equivalent to the van’t hoff factor. Enthalpy is calculated using a calorimeter. For this experiment, it was concluded that as molarity increases that change in temperature increased as well. The change in temperature from the data resulted in a freezing point decrease with is Optimal for a deicer. However, when it came to the Calorimeter results the temperature decreased, leading to believe that NaCl is not the best Deicer. To Conclude the experimnert, NaCl was not a good de-icer due to its endothermic nature in the calorimetry test. The van’t hoff factor for NaCl was ideal. Ultimately the three trials in the calorimetry test lead to the conclusion that NaCl was not a good deicer.
Introduction
This experiment was conducted to determine what the best deicer is to use on Minnesota roads. The tests within the experiment are used to identify whether sodium chloride is a good deicer. The test include a temperature versus molarity where the more the molarity increased the more it impacts the change in freezing point. Another test that was used is a calorimeter, this was done to see the enthalpy of sodium chloride.
It is important as a Midwest state to know what the best deicer to apply to roads is. Throughout the winter months in Minnesota there are multiple times when snowfall results in some form of accident. This could be a minor fender bender or a car crash that leads to someone's death. Although sodium chloride on the road can increase traction on the roads, the environmental impacts on surface water when tested on areas with stream bodies it is worth noting. There is an increase in ‘chloride levels in the downstream section of the streams dramatically1. Despite environmental concerns of sodium chloride and many other deicers in multiple studies deicers continue to make roads more Safe*.* Ice on roads may lead to severe property destruction when citizens slip on the road and total their car. If no deicer is applied or the wrong deicer is applied citizens may have severe monetary and life-threatening consequences that come with a car being totaled.
There is also the aspect of safety, Despite the previously stated negative impacts of deicer's on the environment It is important to realize that they do make the roads in the Midwest more safe to drive on. It is fair to say that one of the benefits of using deicers and sand for increasing safety of winter driving remained a major factor in the continuing use of these snow and ice control materials2.
Eperimimental
For the first part of the experiment 0.1813 m,0.3542 m, 0.5270 m, 0.6844 m and 0.8692 m of solid NaCl were all measured and put into separate test tubes with 10 mL of deionized water via a scoopula. The NaCl was through stirred. An ice bath was created where ice, water and rock salt were used to create a cold mixture. The test tube was then put in the cold solution and the temperature was measured. The initial temperature of the solution was also taken at 0℃. This initial temperature was compared to the temperature after sodium chloride was added to see if the temperature decreased or increased.
For the second experiment The enthalpy of the solution was found. This was done by creating a cowriter with styrofoam cups, cardboard and a temperature prode in the top. A Calorimeter constant was found by mixing hot and cold walk and noting the temperature changes. This was done by combining NaCl and water. The Enthalpy of Dissolution was determined in a calorimeter to determine if the deicer is exothermic or endothermic. In order to support the earlier findings, it was concluded that the results observed were valid and that NaCl is not an accurate deicer by measuring the calorimeter constant. This was done by keeping one set of deionized water at a cold temperature (8.5 ℃) and another set of deionized water was sat on a heat pad. The heat of the water after the heating pad was 92.9℃. The final temperature ended up being 44.1℃. This information was used to calculate the Calorimeter constant. Using this equation (0=qc+qH+qcal=ccalT) where qc is heat of cold water, qH is heat of warm water and qcal is the calorimeter constant and ccalT is the final temperature. When plugging in all number a answer of 77.58J is achieved.
From the newly discovered calorimeter constant a Qrxn can be found. It is important to note that 2 trials were conducted using the equation qrxn = mc h20 +q cal. With the results being an average of 38.6J. This is not what was expected. However, in a separate third experiment the same result was achieved. This means the average heat of displacement was 2.15kJ.
Results
The results for the first experiment were as expected. As molarity increased the change in temperature also increased. It is important to note that the change in temperature was negative leading the deicer to theoretically be more efficient.
Mol/kg (m) | Initial temp (℃) | Final temp (ΔT) | Chang in temp (℃) |
|---|---|---|---|
.181 | 0 | 0.1 | 0.1 |
.354 | 0 | -0.6 | -0.6 |
.527 | 0 | -1.5 | -1.5 |
.684 | 0 | -2 | -2 |
.869 | 0 | -3.7 | -3.7 |
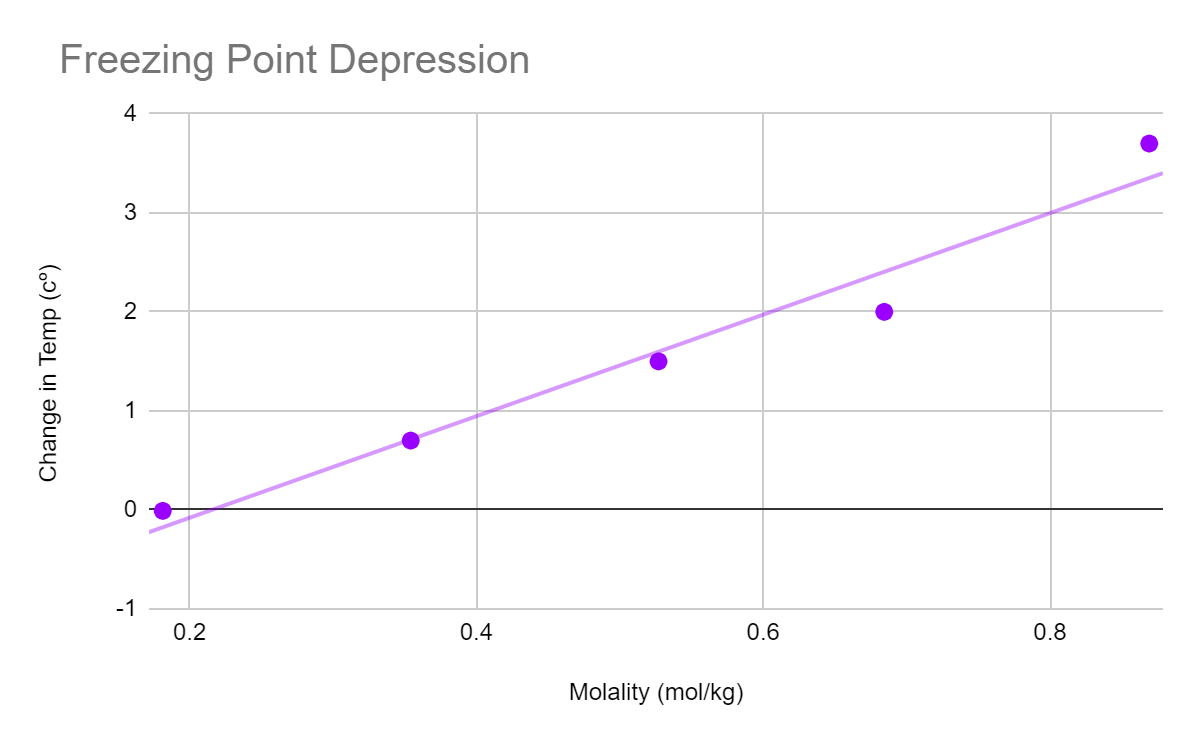
Following the conclusion of each trial, the freezing point was calculated using the molality of NaCl. The data shown above summarizes this point. Later on in the lab the same findings were used to identify the freezing point depression. To illustrate how the freezing points changed in relation to the results for the molality concentrations, these were then displayed on a graph. The van't hoff factor was then calculated to be 2.719 by dividing the slope of the graph by the cryoscopic constant of water, which is 1.86 K mol-1 kg-1.
Hot water Temp(℃) | Cold water Temp(℃) | Difference between hot temp(℃)and cold temp(℃) | Final Temp(℃) | Calorimeter Constant (Joules) |
|---|---|---|---|---|
92.9 | 8.5 | 84.4 | 44.1 | 77.58 |
The data above is used in order to illustrate the difference in hot and cold temperatures of the water. By mixing both of the hot and cold water Solutions into the calorimeter at the same time the difference was greater than 50 degrees celsius and received a calorimeter constant.
Trial Number | Grams (g) of NaCl | Initial temperature (Celsius) | Final temperature (Celsius) | Change in Temperature | Qrxn |
|---|---|---|---|---|---|
1 | 0.995 | 21.8 | 21.3 | -0.5 | |
2 | 1.001 | 20.9 | 20.5 | -0.4 | |
Avarage | .998 | 21.35 | 20.9 | -0.45 | 36.35 |
The following table above shows the main problem with the experiment conducted. When deicer was added to a water solution the temperature went down when it was supposed to go up. The reaction was expected to be exothermic as the temperature of the freezing point was supposed to decrease. This did not happen in the data above. It is important to note that this data is the average of 2 trails on day 2.
This table exhibits a freezing point depression graph that shows the impact of NaCl concentration on the freezing point of water. Upon examining the graph, it is apparent that the temperature of the solution increases with an increase in NaCl concentration. This observed trend is unexpected since the addition of NaCl typically results in a decrease in temperature due to the endothermic process involved in the solution's formation.
Discussion
The results of this experiment have provided us with valuable information regarding the effectiveness of sodium chloride as a deicer. The data we collected showed that sodium chloride was not a great deicer, as the enthalpy of reaction was found to be endothermic. This means that heat is absorbed by the system, resulting in a warmer freezing point for the ice. This is in contrast to our findings in the first experiment, where we observed a decrease in temperature, leading to a lower freezing point.
It is important to understand the chemical structure of an endothermic reaction to fully comprehend our results. During an endothermic reaction, the bonds between sodium and chloride break, forming ions. This breaking of bonds requires energy, and when the ions are floating in water, they also carry energy with them. When the energy required to break the bonds is greater than the energy released when the ions are floating freely, an endothermic reaction occurs.
Our findings have significant implications, as they demonstrate the limitations of using sodium chloride as a deicer. In areas where temperatures regularly drop below freezing, it may be necessary to consider alternative de-icing agents. The information we have gathered can be applied to make informed decisions about road safety during the winter months.
Further research could investigate the effectiveness of other deicers, like calcium chloride or magnesium chloride, to determine if they provide more efficient and effective solutions to the challenges posed by winter weather conditions. It is also worth noting investigating the specific chemical processes involved in the reactions of these different deicers could provide a deeper understanding of their effectiveness in various conditions.
Concussion
The goal of the experiment was to determine whether sodium chloride (NaCl) is an effective deicer for Minnesota roads. The results indicated that as the concentration of NaCl increased, the change in freezing point decreased, which is beneficial for deicing. However, the calorimeter results revealed that the temperature decreased, suggesting that NaCl is not the ideal deicer. Despite the negative environmental impacts associated with deicers, they are necessary to maintain safe road conditions during winter. With this being said, it is important to conduct research on alternative deicers that are both safe for the environment and effective in ensuring road safety. Overall, this experiment provides great insights into the characteristics of NaCl as a deicer and emphasizes the importance of further research about deicers in general.
Work Cited
1.Environmental Impact of Chemical Deicers - A Review. (n.d.). Retrieved from https://www.sciencedirect.com/science/article/pii/S0165232X97000157
2.Fischel, M. (1999). Evaluation of Selected Deicers Based on a Review of the Literature. Transportation Research Record: Journal of the Transportation Research Board, 1662(1), 99-105. doi: 10.3141/1662-13
Lab Report: Deicer
Finding out if NaCl is the best Deicer for Minnesota Roads by Increasing Water's Freezing Point and Observing Dissolution Specific heat
Riley Mills, Angel Doss, Emily Eydel and Matthew Hang
February 28th, Spring 2023
Abstract
In this experiment a variety of tests were performed to find out if Sodium chloride (NaCl) was a good deicer for Minnesota roads. The test that were performed were finding the molarity (x axis) versus the change in freezing point (y axis)and finding the enthalpy of dissolution for NaCl. The graph has a slope that is equivalent to the van’t hoff factor. Enthalpy is calculated using a calorimeter. For this experiment, it was concluded that as molarity increases that change in temperature increased as well. The change in temperature from the data resulted in a freezing point decrease with is Optimal for a deicer. However, when it came to the Calorimeter results the temperature decreased, leading to believe that NaCl is not the best Deicer. To Conclude the experimnert, NaCl was not a good de-icer due to its endothermic nature in the calorimetry test. The van’t hoff factor for NaCl was ideal. Ultimately the three trials in the calorimetry test lead to the conclusion that NaCl was not a good deicer.
Introduction
This experiment was conducted to determine what the best deicer is to use on Minnesota roads. The tests within the experiment are used to identify whether sodium chloride is a good deicer. The test include a temperature versus molarity where the more the molarity increased the more it impacts the change in freezing point. Another test that was used is a calorimeter, this was done to see the enthalpy of sodium chloride.
It is important as a Midwest state to know what the best deicer to apply to roads is. Throughout the winter months in Minnesota there are multiple times when snowfall results in some form of accident. This could be a minor fender bender or a car crash that leads to someone's death. Although sodium chloride on the road can increase traction on the roads, the environmental impacts on surface water when tested on areas with stream bodies it is worth noting. There is an increase in ‘chloride levels in the downstream section of the streams dramatically1. Despite environmental concerns of sodium chloride and many other deicers in multiple studies deicers continue to make roads more Safe*.* Ice on roads may lead to severe property destruction when citizens slip on the road and total their car. If no deicer is applied or the wrong deicer is applied citizens may have severe monetary and life-threatening consequences that come with a car being totaled.
There is also the aspect of safety, Despite the previously stated negative impacts of deicer's on the environment It is important to realize that they do make the roads in the Midwest more safe to drive on. It is fair to say that one of the benefits of using deicers and sand for increasing safety of winter driving remained a major factor in the continuing use of these snow and ice control materials2.
Eperimimental
For the first part of the experiment 0.1813 m,0.3542 m, 0.5270 m, 0.6844 m and 0.8692 m of solid NaCl were all measured and put into separate test tubes with 10 mL of deionized water via a scoopula. The NaCl was through stirred. An ice bath was created where ice, water and rock salt were used to create a cold mixture. The test tube was then put in the cold solution and the temperature was measured. The initial temperature of the solution was also taken at 0℃. This initial temperature was compared to the temperature after sodium chloride was added to see if the temperature decreased or increased.
For the second experiment The enthalpy of the solution was found. This was done by creating a cowriter with styrofoam cups, cardboard and a temperature prode in the top. A Calorimeter constant was found by mixing hot and cold walk and noting the temperature changes. This was done by combining NaCl and water. The Enthalpy of Dissolution was determined in a calorimeter to determine if the deicer is exothermic or endothermic. In order to support the earlier findings, it was concluded that the results observed were valid and that NaCl is not an accurate deicer by measuring the calorimeter constant. This was done by keeping one set of deionized water at a cold temperature (8.5 ℃) and another set of deionized water was sat on a heat pad. The heat of the water after the heating pad was 92.9℃. The final temperature ended up being 44.1℃. This information was used to calculate the Calorimeter constant. Using this equation (0=qc+qH+qcal=ccalT) where qc is heat of cold water, qH is heat of warm water and qcal is the calorimeter constant and ccalT is the final temperature. When plugging in all number a answer of 77.58J is achieved.
From the newly discovered calorimeter constant a Qrxn can be found. It is important to note that 2 trials were conducted using the equation qrxn = mc h20 +q cal. With the results being an average of 38.6J. This is not what was expected. However, in a separate third experiment the same result was achieved. This means the average heat of displacement was 2.15kJ.
Results
The results for the first experiment were as expected. As molarity increased the change in temperature also increased. It is important to note that the change in temperature was negative leading the deicer to theoretically be more efficient.
Mol/kg (m) | Initial temp (℃) | Final temp (ΔT) | Chang in temp (℃) |
|---|---|---|---|
.181 | 0 | 0.1 | 0.1 |
.354 | 0 | -0.6 | -0.6 |
.527 | 0 | -1.5 | -1.5 |
.684 | 0 | -2 | -2 |
.869 | 0 | -3.7 | -3.7 |
Following the conclusion of each trial, the freezing point was calculated using the molality of NaCl. The data shown above summarizes this point. Later on in the lab the same findings were used to identify the freezing point depression. To illustrate how the freezing points changed in relation to the results for the molality concentrations, these were then displayed on a graph. The van't hoff factor was then calculated to be 2.719 by dividing the slope of the graph by the cryoscopic constant of water, which is 1.86 K mol-1 kg-1.
Hot water Temp(℃) | Cold water Temp(℃) | Difference between hot temp(℃)and cold temp(℃) | Final Temp(℃) | Calorimeter Constant (Joules) |
|---|---|---|---|---|
92.9 | 8.5 | 84.4 | 44.1 | 77.58 |
The data above is used in order to illustrate the difference in hot and cold temperatures of the water. By mixing both of the hot and cold water Solutions into the calorimeter at the same time the difference was greater than 50 degrees celsius and received a calorimeter constant.
Trial Number | Grams (g) of NaCl | Initial temperature (Celsius) | Final temperature (Celsius) | Change in Temperature | Qrxn |
|---|---|---|---|---|---|
1 | 0.995 | 21.8 | 21.3 | -0.5 | |
2 | 1.001 | 20.9 | 20.5 | -0.4 | |
Avarage | .998 | 21.35 | 20.9 | -0.45 | 36.35 |
The following table above shows the main problem with the experiment conducted. When deicer was added to a water solution the temperature went down when it was supposed to go up. The reaction was expected to be exothermic as the temperature of the freezing point was supposed to decrease. This did not happen in the data above. It is important to note that this data is the average of 2 trails on day 2.
This table exhibits a freezing point depression graph that shows the impact of NaCl concentration on the freezing point of water. Upon examining the graph, it is apparent that the temperature of the solution increases with an increase in NaCl concentration. This observed trend is unexpected since the addition of NaCl typically results in a decrease in temperature due to the endothermic process involved in the solution's formation.
Discussion
The results of this experiment have provided us with valuable information regarding the effectiveness of sodium chloride as a deicer. The data we collected showed that sodium chloride was not a great deicer, as the enthalpy of reaction was found to be endothermic. This means that heat is absorbed by the system, resulting in a warmer freezing point for the ice. This is in contrast to our findings in the first experiment, where we observed a decrease in temperature, leading to a lower freezing point.
It is important to understand the chemical structure of an endothermic reaction to fully comprehend our results. During an endothermic reaction, the bonds between sodium and chloride break, forming ions. This breaking of bonds requires energy, and when the ions are floating in water, they also carry energy with them. When the energy required to break the bonds is greater than the energy released when the ions are floating freely, an endothermic reaction occurs.
Our findings have significant implications, as they demonstrate the limitations of using sodium chloride as a deicer. In areas where temperatures regularly drop below freezing, it may be necessary to consider alternative de-icing agents. The information we have gathered can be applied to make informed decisions about road safety during the winter months.
Further research could investigate the effectiveness of other deicers, like calcium chloride or magnesium chloride, to determine if they provide more efficient and effective solutions to the challenges posed by winter weather conditions. It is also worth noting investigating the specific chemical processes involved in the reactions of these different deicers could provide a deeper understanding of their effectiveness in various conditions.
Concussion
The goal of the experiment was to determine whether sodium chloride (NaCl) is an effective deicer for Minnesota roads. The results indicated that as the concentration of NaCl increased, the change in freezing point decreased, which is beneficial for deicing. However, the calorimeter results revealed that the temperature decreased, suggesting that NaCl is not the ideal deicer. Despite the negative environmental impacts associated with deicers, they are necessary to maintain safe road conditions during winter. With this being said, it is important to conduct research on alternative deicers that are both safe for the environment and effective in ensuring road safety. Overall, this experiment provides great insights into the characteristics of NaCl as a deicer and emphasizes the importance of further research about deicers in general.
Work Cited
1.Environmental Impact of Chemical Deicers - A Review. (n.d.). Retrieved from https://www.sciencedirect.com/science/article/pii/S0165232X97000157
2.Fischel, M. (1999). Evaluation of Selected Deicers Based on a Review of the Literature. Transportation Research Record: Journal of the Transportation Research Board, 1662(1), 99-105. doi: 10.3141/1662-13
 Knowt
Knowt