
Modern Atomic Theory Review
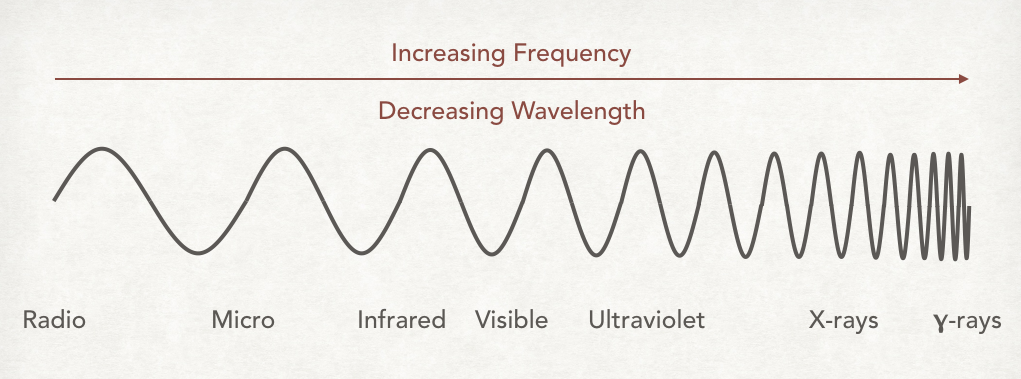
light is a form of electromagnetic radiation
properties of both waves and particles:
wavelength (λ) - the distance between adjacent wave crests, meters
red light (750 NM) has longest wave length
violet light (400 NM) has shortest wave length
1 NM = 1 * 10^-9 meters
frequency (v) - number of cycles or crests that pass through a stationary point in one second
amplitude - the height of the wave from zero to crest
wavelength and frequency are inversely/indirectly related - the shorter the wavelength, the higher the frequency
speed of light: 2.998 * 10^8 meters/second = λv
electromagnetic radiation
light can be viewed as a stream of particles
particle of light is a photon
photon - a single packet of light energy
has specific wavelength, determines what light we see
wavelengths of spectral lines are characteristics of the element
make up atomic emission spectra
no two elements have the same emission spectra
amount of energy carried in the packet depends on the wavelength of the light - the shorter the wavelength, the greater the energy
light waves that carry more energy in their crests are closer
violet light carries more energy per photon than red light
the photoelectric effect - the emission of electrons from a metal when light shines on the metal
quantum of energy - the minimum quantum of energy that can be lost or gained by an atom
quantized: an electron has to absorb/emit a specific amount of energy to move from one energy level to another
ground state: the normal energy level any given electron occupies
excited state: the energy level an electron occupies when it has absorbed the specific quantum of energy to move up to that level
Planck’s Law - E=Hv
E - energy, joules
H - Planck’s constant, 6.626 * 10^-34 J*S
v - frequency
Bohr’s Model
Niels Bohr changed Rutherford’s model to include newer discoveries about how the energy of an atom changes when the atom absorbs/emits energy
proposed electron is found only in specific circular paths/orbits around the nucleus ❌
incorrect - if the orbits were truly circular, the electron would spiral into the nucleus
each possible electron orbit has a fixed energy - energy level ✅
each orbit is a specific distance from the nucleus and at each specific energy
impossible for an electron to exist between orbits
amount of energy is directly related to the frequency → wavelength
de Broglie: proposed “electrons be considered as waves confined to the space around an atomic nucleus”
Heisenberg Uncertainty Principle
Werner Heisenberg
states that it is impossible to determine simultaneously both the position and velocity of an electron
“we cannot know both the position and speed of a particle, such as a photon or electron, with perfect accuracy”
Schrödinger Wave Equation
Erwin Schrödinger developed an equation that treated electrons as waves
Quantum Theory - describes mathematically the wave properties of electrons
electrons exist in certain regions called orbitals
orbitals - 3D regions around the nucleus that indicate the probable location of an electron
represent probability maps showing a statistical attribution of where the electron is likely to be found
4 Wave Properties
Energy Level: Principal Quantum Numbers - number specifying the principle shell of orbital
n - indicates the energy level
energy increases with principal quantum number
maximum of 7 energy levels
n^2 - how many orbitals in any energy level
2n^2 - maxim. number of electrons possible in any energy level
Sub Level: Shapes of Quantum Mechanical Orbitals
letter indicates subshell of orbital, specifies shape
possible letters - s, p, d, f
electrons are more likely to be found closer to the nucleus than farther away
Orbital: Orientation
s - 1 orbital
p - 3 orbitals
d - 5 orbitals
f - 7 orbitals
Spin: clockwise or counterclockwise
ENERGY LEVEL | SUB-LEVEL | # ORBITALS (n^2) | ELECTRONS (2n^2) |
|---|---|---|---|
n=1 | 1s | 1 | 2 |
n=2 | 2s 2p | 4 | 8 |
n=3 | 3s 3p 3d | 9 | 18 |
n=4 | 4s 4p 4d 4f | 16 | 32 |
Electron Configuration
arrangement of electrons in an atom and the way in which the electrons are arranged in various orbitals around the nucleus
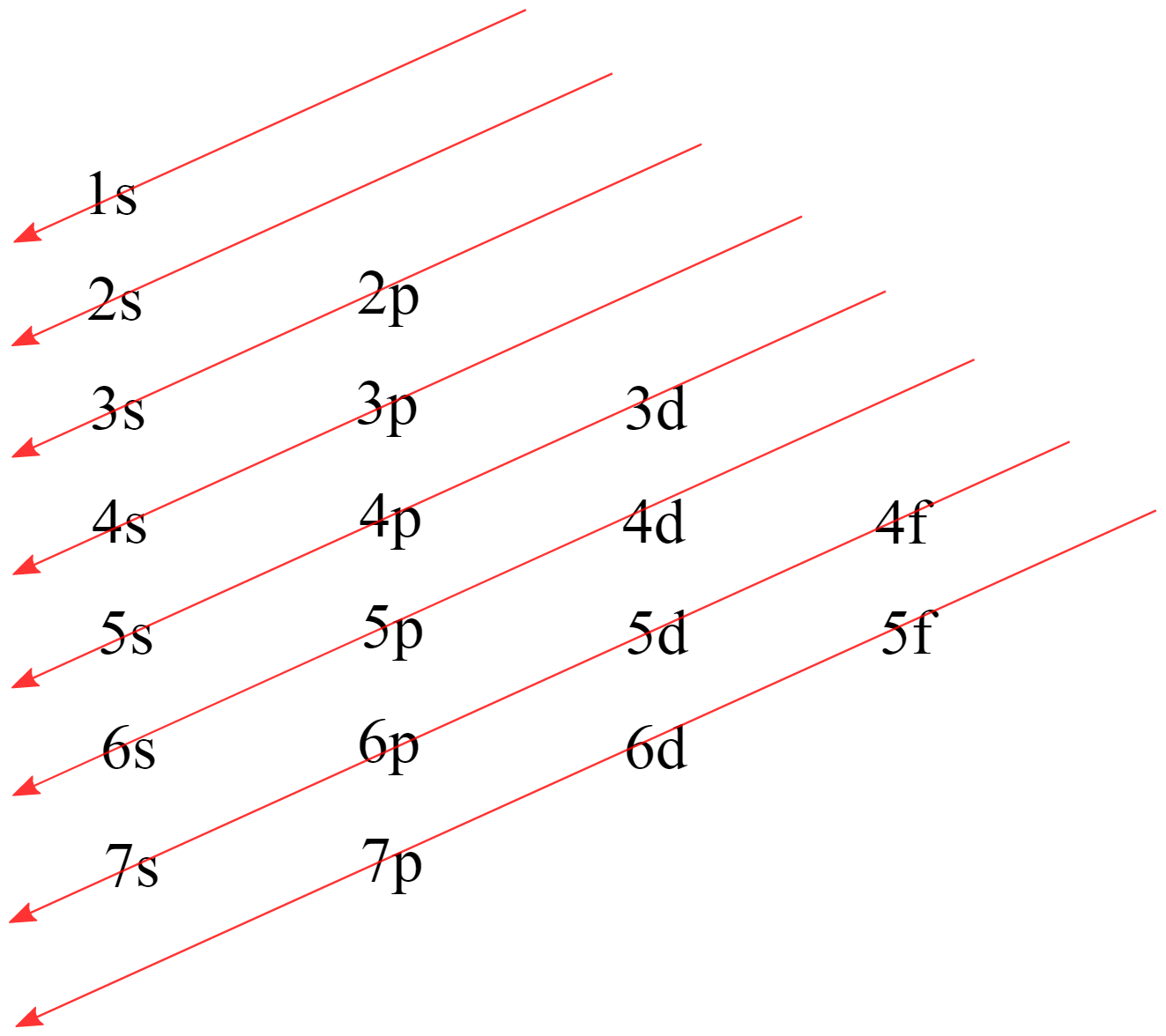
Aufbau Principle
the electrons will fill the orbitals in a very specific order
lowest → highest energy
The Diagonal Rule
Pauli Exclusion Principle
an individual orbital may describe at most TWO electrons
in order to occupy the orbital, the two electrons must have opposite spins: ⬆⬇
EXAMPLES
Carbon 6e- : 1s^2, 2s^2, 2p^2
Aluminum 13e- : 1s^2, 2s^2, 2p^6, 3s^2, 3p^1
Noble Gas Configuration
Aluminum 13e- : [Ne] 3s^2, 3p^1
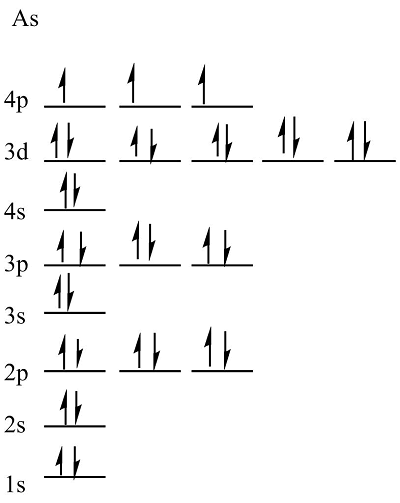
Hund’s Rule
orbitals in the same sub-level must all fill with one electron before a second electron is added to any of the orbitals: (n)p^4 - ⬆⬇ ⬆ ⬆
the “single” electrons will all have the same spin direction
Orbital Diagram
Valence and Core Electrons
valence electrons are the electrons on the outermost energy level
the noble gases always have full valence shells
Selenium 34e- : 1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 3d^10, 4s^2, 3d^10, 4p^4
Silicon 14e- : 1s^2, 2s^2, 2p^6, 3s^2, 3p^2
the chemical properties of elements are largely determined by the number of valence electrons they contain
properties vary in a periodic fashion because the number of valence electrons is periodic
Atomic Physical Properties
Atomic Size ⬇⬅
left-right decreases ⬅
across a period, the amount of protons in the nucleus increases which has a stronger pull on the electrons, causing them to move closer to the nucleus
top-bottom increases ⬇
size of the orbital increases with increasing principal quantum shell number
electrons occupying the outermost orbitals are farthest from nucleus
Ionization Energy ⬆➡
amount of energy needed to remove a single electron
left-right increases ➡
electrical pull on electrons from the # protons in nucleus causes increases amount of energy needed
top-bottom decreases ⬆
electrons in outermost orbitals are less affected by the electrical pull from nucleus
Electronegativity ⬆➡
ability of an atom of an element to attract electrons when the atom is in a compound
left-right increases ➡
top-bottom decreases ⬆
Modern Atomic Theory Review
light is a form of electromagnetic radiation
properties of both waves and particles:
wavelength (λ) - the distance between adjacent wave crests, meters
red light (750 NM) has longest wave length
violet light (400 NM) has shortest wave length
1 NM = 1 * 10^-9 meters
frequency (v) - number of cycles or crests that pass through a stationary point in one second
amplitude - the height of the wave from zero to crest
wavelength and frequency are inversely/indirectly related - the shorter the wavelength, the higher the frequency

speed of light: 2.998 * 10^8 meters/second = λv
electromagnetic radiation
light can be viewed as a stream of particles
particle of light is a photon
photon - a single packet of light energy
has specific wavelength, determines what light we see
wavelengths of spectral lines are characteristics of the element
make up atomic emission spectra
no two elements have the same emission spectra
amount of energy carried in the packet depends on the wavelength of the light - the shorter the wavelength, the greater the energy
light waves that carry more energy in their crests are closer
violet light carries more energy per photon than red light
the photoelectric effect - the emission of electrons from a metal when light shines on the metal
quantum of energy - the minimum quantum of energy that can be lost or gained by an atom
quantized: an electron has to absorb/emit a specific amount of energy to move from one energy level to another
ground state: the normal energy level any given electron occupies
excited state: the energy level an electron occupies when it has absorbed the specific quantum of energy to move up to that level
Planck’s Law - E=Hv
E - energy, joules
H - Planck’s constant, 6.626 * 10^-34 J*S
v - frequency
Bohr’s Model
Niels Bohr changed Rutherford’s model to include newer discoveries about how the energy of an atom changes when the atom absorbs/emits energy
proposed electron is found only in specific circular paths/orbits around the nucleus ❌
incorrect - if the orbits were truly circular, the electron would spiral into the nucleus
each possible electron orbit has a fixed energy - energy level ✅
each orbit is a specific distance from the nucleus and at each specific energy
impossible for an electron to exist between orbits
amount of energy is directly related to the frequency → wavelength
de Broglie: proposed “electrons be considered as waves confined to the space around an atomic nucleus”
Heisenberg Uncertainty Principle
Werner Heisenberg
states that it is impossible to determine simultaneously both the position and velocity of an electron
“we cannot know both the position and speed of a particle, such as a photon or electron, with perfect accuracy”
Schrödinger Wave Equation
Erwin Schrödinger developed an equation that treated electrons as waves
Quantum Theory - describes mathematically the wave properties of electrons
electrons exist in certain regions called orbitals
orbitals - 3D regions around the nucleus that indicate the probable location of an electron
represent probability maps showing a statistical attribution of where the electron is likely to be found
4 Wave Properties
Energy Level: Principal Quantum Numbers - number specifying the principle shell of orbital
n - indicates the energy level
energy increases with principal quantum number
maximum of 7 energy levels
n^2 - how many orbitals in any energy level
2n^2 - maxim. number of electrons possible in any energy level
Sub Level: Shapes of Quantum Mechanical Orbitals
letter indicates subshell of orbital, specifies shape
possible letters - s, p, d, f
electrons are more likely to be found closer to the nucleus than farther away
Orbital: Orientation
s - 1 orbital
p - 3 orbitals
d - 5 orbitals
f - 7 orbitals
Spin: clockwise or counterclockwise
ENERGY LEVEL | SUB-LEVEL | # ORBITALS (n^2) | ELECTRONS (2n^2) |
|---|---|---|---|
n=1 | 1s | 1 | 2 |
n=2 | 2s 2p | 4 | 8 |
n=3 | 3s 3p 3d | 9 | 18 |
n=4 | 4s 4p 4d 4f | 16 | 32 |
Electron Configuration
arrangement of electrons in an atom and the way in which the electrons are arranged in various orbitals around the nucleus
Aufbau Principle
the electrons will fill the orbitals in a very specific order
lowest → highest energy
The Diagonal Rule

Pauli Exclusion Principle
an individual orbital may describe at most TWO electrons
in order to occupy the orbital, the two electrons must have opposite spins: ⬆⬇
EXAMPLES
Carbon 6e- : 1s^2, 2s^2, 2p^2
Aluminum 13e- : 1s^2, 2s^2, 2p^6, 3s^2, 3p^1
Noble Gas Configuration
Aluminum 13e- : [Ne] 3s^2, 3p^1
Hund’s Rule
orbitals in the same sub-level must all fill with one electron before a second electron is added to any of the orbitals: (n)p^4 - ⬆⬇ ⬆ ⬆
the “single” electrons will all have the same spin direction
Orbital Diagram

Valence and Core Electrons
valence electrons are the electrons on the outermost energy level
the noble gases always have full valence shells
Selenium 34e- : 1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 3d^10, 4s^2, 3d^10, 4p^4
Silicon 14e- : 1s^2, 2s^2, 2p^6, 3s^2, 3p^2
the chemical properties of elements are largely determined by the number of valence electrons they contain
properties vary in a periodic fashion because the number of valence electrons is periodic
Atomic Physical Properties
Atomic Size ⬇⬅
left-right decreases ⬅
across a period, the amount of protons in the nucleus increases which has a stronger pull on the electrons, causing them to move closer to the nucleus
top-bottom increases ⬇
size of the orbital increases with increasing principal quantum shell number
electrons occupying the outermost orbitals are farthest from nucleus
Ionization Energy ⬆➡
amount of energy needed to remove a single electron
left-right increases ➡
electrical pull on electrons from the # protons in nucleus causes increases amount of energy needed
top-bottom decreases ⬆
electrons in outermost orbitals are less affected by the electrical pull from nucleus
Electronegativity ⬆➡
ability of an atom of an element to attract electrons when the atom is in a compound
left-right increases ➡
top-bottom decreases ⬆
 Knowt
Knowt
