AP BIO CHAPTER 7: Cellular Respiration and Fermentation
Introduction
7.1 Catabolic pathways yield energy by oxidizing organic fuels
7.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate
7.3 After pyruvate is oxidized, the citric acid cycle completes the energy-yielding oxidation of organic molecules
7.4 During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis
7.5 Fermentation and anaerobic respiration enable cells to produce ATP without the use of oxygen
7.6 Glycolysis and the citric acid cycle connect to many other metabolic pathways
BIG IDEAS: Heterotrophic organisms must capture free energy from their environment to maintain life (Big Idea 2). Common to all are core processes that move energy and matter through living systems and thereby support life (Big Ideas 1 & 4).
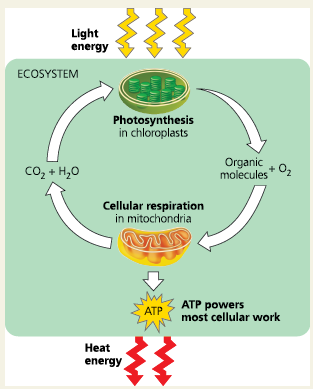
Photosynthesis vs Respiration
Photosynthesis generates oxygen and organic molecules. Respiration breaks down this fuel.
Energy flow in an ecosystem
Key Pathways:
Glycolysis
Pyruvate Oxidation
Citric Acid Cycle
Oxidative Phosphorylation
Fermentation
Concept 7.1: Catabolic pathways yield energy by oxidizing organic fuels
Cells break down glucose and other organic fuels to yield chemical energy in the form of ATP. Fermentation is a process that results in the partial degradation of glucose without the use of oxygen. Cellular respiration is a more complete breakdown of glucose; in aerobic respiration, oxygen is used as a reactant. The cell taps the energy stored in food molecules through redox reactions**, in which one substance partially or totally shifts electrons to another.** Oxidation is the loss of electrons from one substance, while reduction is the addition of electrons to the other.
During aerobic respiration, glucose (C6H12O6) is oxidized to CO2, and O2 is reduced to H2O. Electrons lose potential energy during their transfer from glucose or other organic compounds to oxygen. Electrons are usually passed first to NAD+, reducing it to NADH, and then from NADH to an electron transport chain, which conducts them to O2 in energy-releasing steps. The energy is used to make ATP.
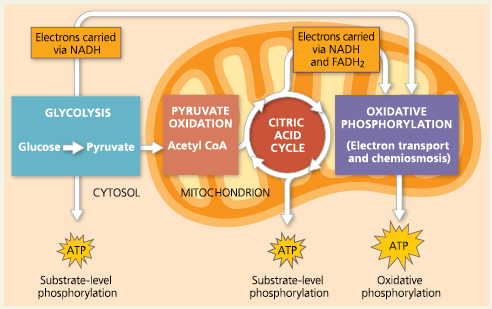
Aerobic respiration occurs in three stages: (1) glycolysis, (2) pyruvate oxidation and the citric acid cycle, and (3) oxidative phosphorylation (electron transport and chemiosmosis).
What plays a major role in catabolic pathways?
Electron transfer
Cells degrade organic molecules with high potential energy →
energy to do work, some dissipated as heat
Fermentation:
A catabolic process that makes a limited amount of ATP from glucose (or other organic molecules) without an electron transport chain or the use of oxygen and that produces a characteristic end product, such as ethyl alcohol or lactic acid
Aerobic Respiration:
A catabolic pathway for organic molecules, using O2 as the final electron acceptor in an electron transport chain and ultimately producing ATP. (Oxygen and organic fuel consumed as reactant)
Is aerobic respiration efficient, and where is it carried out?
It is the most efficient catabolic pathway and is carried out in most eukaryotic cells and many prokaryotic organisms.
Anaerobic Respiration:
Some prokaryotes use substances other than oxygen as reactants in a similar process that harvests chemical energy without oxygen (the prefix an- means “without”).
Cellular Respiration:
The catabolic pathways of aerobic and anaerobic respiration break down organic molecules and use an electron transport chain for the production of ATP.
_____ is often used to refer to the aerobic process
cellular respiration
Oxidation-Reduction Reactions / Redox Reactions:
A chemical reaction involving the complete or partial transfer of one or more electrons from one reactant to another
Oxidation:
The complete or partial loss of electrons from a substance involved in a redox reaction
Reduction:
The complete or partial addition of electrons to a substance involved in a redox reaction (Note that adding electrons is called reduction; adding negatively charged electrons to an atom reduces the amount of positive charge of that atom.)
Redox Reaction
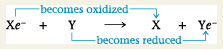
Reducing Agent:
The electron donor in a redox reaction
Oxidizing Agent:
The electron acceptor in a redox reaction
Why would a reaction release energy to the surroundings?
The electrons lose potential energy when they end up being shared unequally, spending more time near electronegative atoms such as oxygen
Methane Combustion
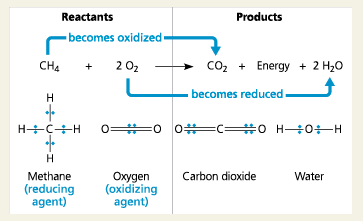
Because oxygen is so ____, it is one of the most powerful of all oxidizing agents.
electronegative
____ must be added to pull an electron away from an atom.
energy
Electronegativity:
How an atom pulls on electrons (higher electronegativity = more energy requires to take an electron away from it)
As in the combustion of methane or gasoline…
The fuel (glucose) is oxidized and oxygen is reduced. The electrons lose potential energy along the way, and energy is released.
Why are organic molecules with an abundance of hydrogen excellent fuels?
Their bonds are a source of “hilltop” electrons, whose energy may be released as these electrons “fall” down an energy gradient when they are transferred to oxygen
Only ___ holds back the flood of electrons to a lower energy state.
the barrier of activation energy
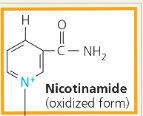
NAD+ (Nicotinamide Adenine Dinucleotide):
A coenzyme that cycles easily between oxidized and reduced (NAD+ and NADH) states, thus acting as an electron carrier
How does NAD+ trap electrons from glucose and other organic molecules in food?
Enzymes called dehydrogenases remove a pair of hydrogen atoms (2 electrons and 2 protons) from the substrate (glucose, in this example), thereby oxidizing it. The enzyme delivers the 2 electrons along with 1 proton to its coenzyme, NAD+, forming NADH (Figure 7.4). The other proton is released as a hydrogen ion (H+) into the surrounding solution
Nicotinamide (oxidized form)
Nicotinamide (reduced form)
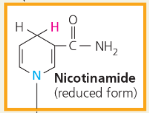
Each NADH molecule formed during respiration represents ___ that can be tapped to make ATP when the electrons complete their “fall” down an energy gradient
stored energy
In cellular respiration, the hydrogen that reacts with oxygen is derived from ___ rather than H2
organic molecules
Instead of occurring in one explosive reaction, respiration uses an electron transport chain to break the fall of electrons to oxygen into ___
several energy-releasing steps
Electron Transport Chain:
A sequence of electron carrier molecules (membrane proteins) that shuttle electrons down a series of redox reactions that release energy used to make ATP
Where are electron transport chains?
They are in the inner membrane of the mitochondria of eukaryotic cells and the plasma membrane of aerobically respiring prokaryotes
Electrons removed from glucose are shuttled by NADH to the ___ end of the chain. At the _ end, O2 captures these electrons along with hydrogen nuclei (H+), forming water.
top/higher-energy; bottom/lower-energy
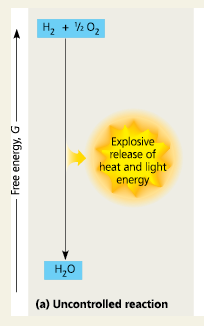
An explosion
The one-step exergonic reaction of hydrogen with oxygen to form water releases a large amount of energy in the form of heat and light
Uncontrolled Reaction
Cellular Respiration
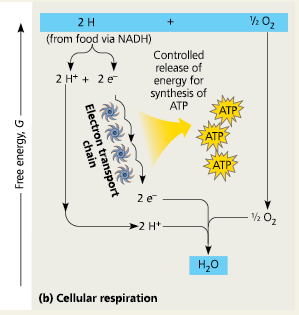
Electron transfer from NADH to oxygen is an ___ reaction
exergonic
Each “downhill” carrier is more ___ than and thus capable of oxidizing, its “uphill” neighbor, with oxygen at the bottom of the chain.
electronegative
Downhill Route
glucose → NADH → electron transport chain → oxygen
The Stages of Cellular Respiration: A Preview
Glycolysis:
A series of reactions that ultimately splits glucose into pyruvate, Glycolysis occurs in almost all living cells, serving as the starting point for fermentation or cellular respiration.
Where does glycolysis occur?
It occurs in the cytosol
___ begins the degradation process by breaking glucose into two molecules of a compound called pyruvate
Glycolysis
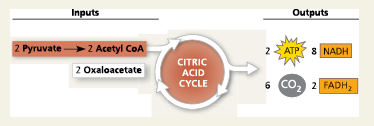
Citric Acid Cycle (Krebs Cycle):
A chemical cycle involving eight steps that completes the metabolic breakdown of glucose molecules begun in glycolysis by oxidizing acetyl CoA (derived from pyruvate) to carbon dioxide
Where does the citric acid cycle occur?
It occurs in the mitochondrion in eukaryotic cells and in the cytosol of prokaryotes.
The citric acid cycle with ___ is the second major stage in cellular respiration
pyruvate oxidation
In eukaryotes, ___ enters the mitochondrion and is oxidized to a compound called acetyl CoA, which enters the citric acid cycle
pyruvate
When compounds lose electrons, they ___ energy; when compounds gain electrons, they _ energy.
lose; gain
Show the normal, downhill route most electrons follow in cellular respiration.
Glucose → NADH → ETC → Oxygen
Figure 7.6 An overview of cellular respiration
During oxidative phosphorylation, electron transport chains convert the chemical energy to a form used for ATP synthesis in the process called ___
chemiosmosis
Some of the steps of glycolysis and the citric acid cycle are redox reactions in which dehydrogenases transfer electrons from substrates to NAD+, forming ___
NADH
In the third stage of respiration, the electron transport chain ___ (most often via NADH) from the breakdown products of the first two stages and passes these electrons from one molecule to another. At the end of the chain, the electrons are combined with molecular oxygen and hydrogen ions (H+), forming water (see Figure 7.5b).
accepts electrons
The energy released at each step of the chain is stored in a form that the mitochondrion (or prokaryotic cell) and can be used to make ___
ATP from ADP
Oxidative Phosphorylation:
The production of ATP using energy derived from the redox reactions of an electron transport chain; the third major stage of cellular respiration
In eukaryotic cells, ___ is the site of electron transport and chemiosmosis, the processes that together constitute oxidative phosphorylation. (In prokaryotes, these processes take place in the plasma membrane.)
the inner membrane of the mitochondrion
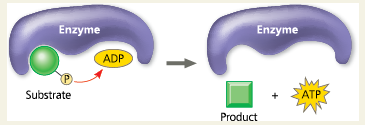
Substrate-Level Phosphorylation:
The enzyme-catalyzed formation of ATP by direct transfer of a phosphate group to ADP from an intermediate substrate in catabolism
Figure 7.7 Substrate-Level Phosphorylation
Review Figure 6.8. Do you think the potential energy is higher for the reactants or the products in the reaction shown above? Explain.
Because there is no external source of energy for the reaction, it must be exergonic, and the reactants must be at a higher energy level than the products.
Compare and contrast aerobic and anaerobic respiration.
Both processes include glycolysis, the citric acid cycle, and oxidative phosphorylation. In aerobic respiration, the final electron acceptor is molecular oxygen (O2); in anaerobic respiration, the final electron acceptor is a different substance.
Name and describe the two ways in which ATP is made during cellular respiration. During what stage(s) in the process does each type occur?
Substrate-level phosphorylation, which occurs during glycolysis and the citric acid cycle, involves the direct transfer of a phosphate group from an organic substrate to ADP by an enzyme. The process of oxidative phosphorylation occurs during the third stage of cellular respiration, which is called oxidative phosphorylation. In this process, the synthesis of ATP from ADP and inorganic phosphate (℗i) is powered by the redox reactions of the electron transport chain.
WHAT IF? If the following redox reaction occurred, which compound would be oxidized? Which reduced? C4H6O5 + NAD+ → C4H4O5 + NADH + H+
C4H6O5 would be oxidized, and NAD+ would be reduced.
Describe the difference between the two processes in cellular respiration that produce ATP: oxidative phosphorylation and substrate-level phosphorylation.
Most of the ATP produced in cellular respiration comes from oxidative phosphorylation, in which the energy released from redox reactions in an electron transport chain is used to produce ATP. In substrate-level phosphorylation, an enzyme directly transfers a phosphate group to ADP from an intermediate substrate. All ATP production in glycolysis occurs by substrate-level phosphorylation; this form of ATP production also occurs at one step in the citric acid cycle.
Concept 7.2: Glycolysis harvests chemical energy by oxidizing glucose to pyruvate
Glycolysis (“splitting of sugar”) is a series of reactions that breaks down glucose into two pyruvate molecules, which may go on to enter the citric acid cycle, and nets 2 ATP and 2 NADH per glucose molecule.
What does glycolysis mean?
“sugar splitting“
The starting product of glycolysis is the six-carbon sugar ___, and the ending product is two three-carbon compounds termed _
glucose; pyruvate
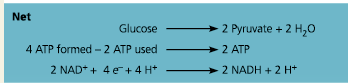
What is the net energy yield from glycolysis, per glucose molecule?
2 ATP plus 2 NADH
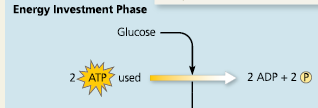
Energy Investment Phase
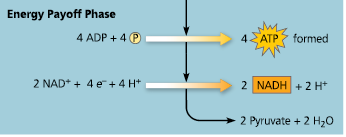
Energy Payoff Phase
Net
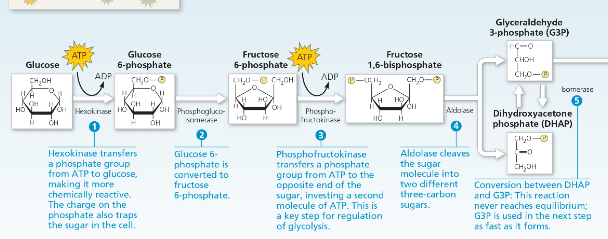
First 5 Steps of Glycolysis
Last 5 Steps of Glycolysis
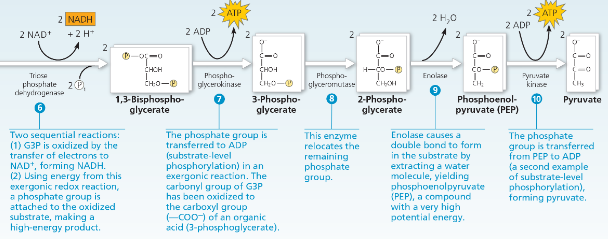
What would happen if you removed the dihydroxyacetone phosphate generated in step 4 as fast as it was produced?
The removal would probably stop glycolysis, or at least slow it down, since it would push the equilibrium for step 5 toward the bottom (toward DHAP). If less (or no) glyceraldehyde 3-phosphate were available, step 6 would slow down (or be unable to occur).
What two stages use ATP in glycolysis?
Stages #1 and #3
What stage forms ATP in glycolysis?
Stage #7
What stage forms NADH in glycolysis?
Stage #6
If ___ is present, the chemical energy stored in pyruvate and NADH can be extracted by pyruvate oxidation, the citric acid cycle, and oxidative phosphorylation
O2
Glycolysis occurs in the ___ of the cell
cytosol
During step 6 in Figure 7.9, which molecule acts as the oxidizing agent? The reducing agent?
NAD+ acts as the oxidizing agent in step 6, accepting electrons from glyceraldehyde 3-phosphate (G3P), which thus acts as the reducing agent.
Which reactions are the source of energy for the formation of ATP and NADH in glycolysis?
The oxidation of the three-carbon sugar glyceraldehyde 3-phosphate yields energy. In this oxidation, electrons and H+ are transferred to NAD+, forming NADH, and a phosphate group is attached to the oxidized substrate. ATP is then formed by substrate-level phosphorylation when this phosphate group is transferred to ADP.
Concept 7.3: After pyruvate is oxidized, the citric acid cycle completes the energy-yielding oxidation of organic molecules
In eukaryotic cells, pyruvate enters the mitochondrion and is oxidized to acetyl CoA, which is further oxidized in the citric acid cycle
Acetyl CoA:
Acetyl coenzyme A: the entry compound for the citric acid cycle in cellular respiration, formed from a two-carbon fragment of pyruvate attached to a coenzyme
Overview of pyruvate oxidation and the citric acid cycle
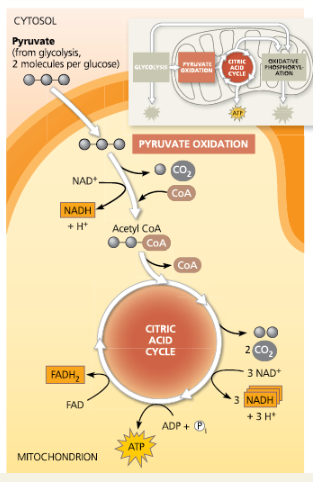
A closer look at the citric acid cycle
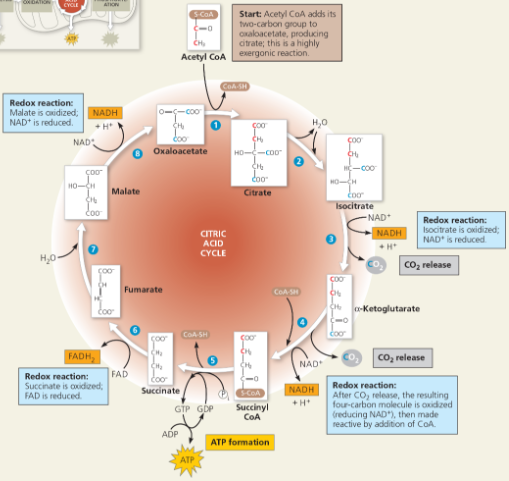
To enter the citric acid cycle, pyruvate must enter the mitochondria by active transport. Three things are necessary to convert pyruvate to acetyl CoA. Explain the 3 steps.
Carbon is taken out and later released and CO2, NAD+ turns into NADH, and CoA joins C-C to make acetyl
How many times does the citric acid cycle occur for each molecule of glucose?
Twice, once per acetyl CoA
Referring to Figure 7.11, how many NADHs are formed?
3
How many carbons are lost as pyruvate is oxidized?
2
What are the carbons released as?
Carbon dioxide
How many FADH2 have been formed?
1
How many ATPs have been formed?
1
How do you find the number of molecules formed from a single glucose?
Since glucose requires two cycles, double the amount of molecules formed from one cycle
The step that converts pyruvate to acetyl CoA at the top of the diagram also occurs twice per glucose. This step accounts for two additional reduced ___ molecules and two carbon dioxide molecules.
NADH
Name the molecules that conserve most of the energy from redox reactions of the citric acid cycle. How is this energy converted to a form that can be used to make ATP?
NADH and FADH2; they will donate electrons to the electron transport chain
What processes in your cells produce the CO2 that you exhale?
CO2 is released from the pyruvate that is the end product of glycolysis, and CO2 is also released during the citric acid cycle.
What molecular products indicate the complete oxidation of glucose during cellular respiration?
The release of six molecules of CO2 represents the complete oxidation of glucose. During the processing of two pyruvates to acetyl CoA, the fully oxidized carboxyl groups (—COO–) are given off as 2 CO2. The remaining four carbons are released as CO2 in the citric acid cycle as citrate is oxidized back to oxaloacetate.
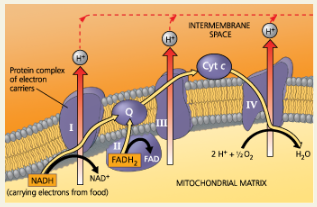
Concept 7.4: During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis
NADH and FADH2 transfer electrons to the electron transport chain. Electrons move down the chain, losing energy in several energy-releasing steps. Finally, electrons are passed to O2, reducing it to H2O.
Along the electron transport chain, electron transfer causes protein complexes to move H+ from the mitochondrial matrix (in eukaryotes) to the intermembrane space, storing energy as a proton-motive force (H+ gradient). As H+ diffuses back into the matrix through ATP synthase, its passage drives the phosphorylation of ADP, an energy-coupling mechanism called chemiosmosis.
About 34% of the energy stored in a glucose molecule is transferred to ATP during cellular respiration, producing a maximum of about 32 ATP.
Prosthetic Groups:
Nonprotein components essential for the catalytic functions of certain enzymes; they are tightly bound to ETC proteins
Electron carriers ___ between reduced and oxidized states as they accept and then donate electrons
alternate
Each component of the chain becomes ___ when it accepts electrons from its “uphill” neighbor, which has a lower affinity for electrons (is less electronegative). It then returns to its _ form as it passes electrons to its “downhill,” more electronegative neighbor.
reduced; oxidized
Free-energy change during electron transport
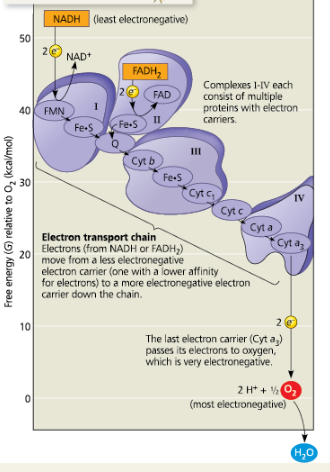
Each member of the electron transport chain is lower in ___ than the preceding member of the chain but higher in _.
free energy; electronegativity
The molecule at zero free energy, which is ___, is the lowest of all the molecules in free energy and highest in electronegativity.
O2
Oxygen stabilizes the electrons by combining with ___ to form _
two hydrogen ions, H2O
The two electron carriers molecules that feed electrons into the electron transport system are ___
NADH and FADH2
Cytochromes:
An iron-containing protein that is a component of electron transport chains in the mitochondria and chloroplasts of eukaryotic cells and the plasma membranes of prokaryotic cells
Heme Group:
A cytochrome’s prosthetic group; has an iron atom that accepts and donates electrons
The last cytochrome of the chain, Cyt a3, passes its electrons to oxygen, which is ___ electronegative
very
Although NADH and FADH2 each donate an equivalent number of electrons (2) for oxygen reduction, the electron transport chain provides about one-third less energy for ATP synthesis when the electron donor is ___
FADH2 rather than NADH
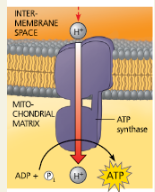
ATP Synthase:
A complex of several membrane proteins that functions in chemiosmosis with adjacent electron transport chains, using the energy of a hydrogen ion (proton) concentration gradient to make ATP.
Where are ATP synthases found?
ATP synthases are found in the inner mitochondrial membranes of eukaryotic cells and in the plasma membranes of prokaryotes.
Chemiosmosis:
An energy-coupling mechanism that uses energy stored in the form of a hydrogen ion gradient across a membrane to drive cellular work, such as the synthesis of ATP.
Under aerobic conditions, most ATP synthesis in cells occurs by ___
chemiosmosis
ATP synthase, a molecular mill: (a) The ATP synthase protein complex functions as a mill, powered by the flow of hydrogen ions.
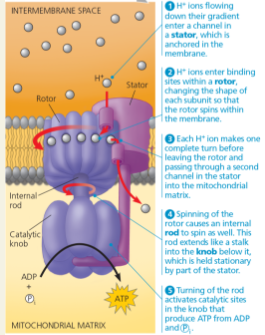
ATP synthase, a molecular mill: (b) This computer model shows the four parts of ATP synthase. Each part consists of a number of polypeptide subunits. The entire structure of the gray region has not yet been determined and is an area of active research.
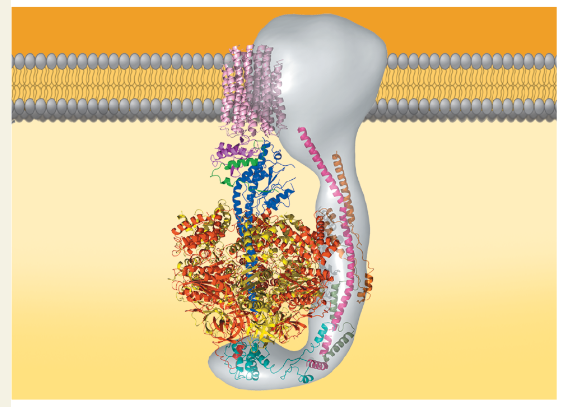
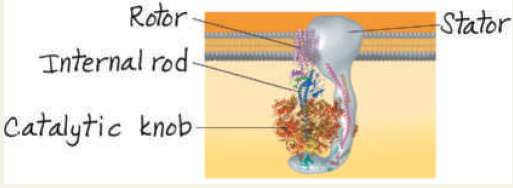
Label the rotor, stator, internal rod, and catalytic knob in part (b), the computer model.
Explain the overall concept of how ATP synthase uses the flow of H+ ions to produce ATP
H+ ions flow down their gradient which turns a rod like a mill. This activates catalytic sites that produce ATP from ADP and Pi.
Chemiosmosis couples the electron transport chain to ATP synthesis
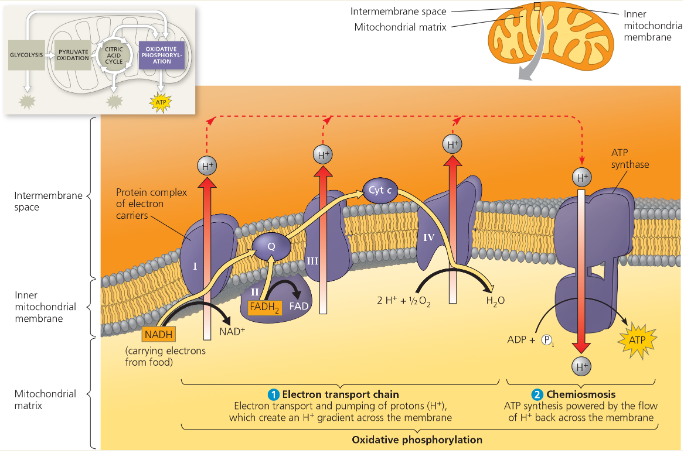
What is the role of the ETC in forming the H+ gradient across the inner mitochondrial membrane?
As NADH and FADH2 deposit electrons to the ETC, NAD+ and FAD+ are formed, and H+ ions are pumped to the inner membrane space. This creates a gradient for H+ to flow back through the ATP synthase
How do chemiosmosis and proton-motive force relate to oxidative phosphorylation?
ATP synthesis is powered by chemiosmosis, using an H+ gradient to drive work. They flow with the proton-motive force, potential energy in the H+ gradient.
If complex IV were nonfunctional, could chemiosmosis produce any ATP, and if so, how would the rate of synthesis differ?
At first, some ATP could be made, since electron transport could proceed as far as complex III, and a small H+ gradient could be built up. Soon, however, no more electrons could be passed to complex III because it could not be reoxidized by passing its electrons to complex IV.
Proton-Motive Force:
The potential energy stored in the form of a proton electrochemical gradient generated by the pumping of hydrogen ions (H+) across a biological membrane during chemiosmosis
Chemiosmosis is an energy-coupling mechanism that uses energy stored in the form of an ___ to drive cellular work
H+gradient across a membrane
What sequence does most energy flow in during respiration?
glucose → NADH → electron transport chain → proton-motive force → ATP
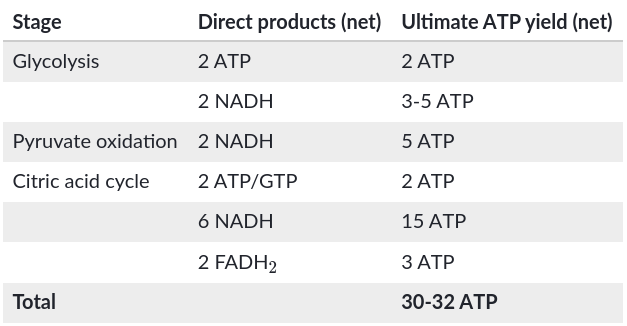
ATP yield per molecule of glucose at each stage of cellular respiration
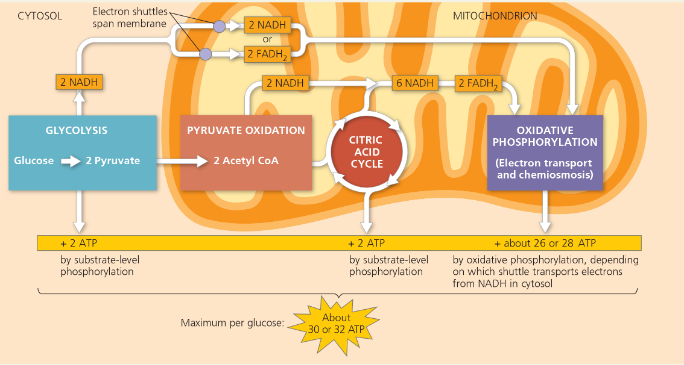
Explain exactly how the total of 26 or 28 ATP (see the yellow bar) was calculated
First, there are 2 NADH from the oxidation of pyruvate plus 6 NADH from the citric acid cycle (CAC); 8 NADH × 2.5 ATP/NADH = 20 ATP. Second, there are 2 FADH2 from the CAC; 2 FADH2 × 1.5 ATP/FADH2 = 3 ATP. Third, the 2 NADH from glycolysis enter the mitochondrion through one of two types of shuttles. They pass their electrons either to 2 FAD, which becomes FADH2 and results in 3 ATP or 2 NAD+, which becomes NADH and results in 5 ATP. Thus, 20 + 3 + 3 = 26 ATP or 20 + 3 + 5 = 28 ATP from all NADH and FADH2.
How many maximum molecules of ATP are formed from NADH and FADH2?
1 NADH results in 10 H+ being pumped, and it takes about 4 H+ for one ATP. That means 1 NADH results in about 2.5 ATP molecules. Since FADH2’s electrons enter the chain later, it only transfers enough H+ to the inner membrane space for about 1.5 ATP.
List the products in glycolysis
2 NADH, 2 Pyruvate, 2 ATP
List the products in pyruvate oxidation
2 Acetyl CoA, 2 NADH, 2 CO2
List the products in the citric acid cycle per one glucose/two turns
4 CO2, 6 NADH, 2 ATP, 2 FADH2
Why is the total count about 36 or 38 ATP molecules rather than a specific number?
The 2 NADH from glycolysis enter the mitochondrion through one of two types of shuttles. They pass their electrons either to 2 FAD, which becomes FADH2 and results in 3 ATP or 2 NAD+ which becomes NADH and results in 5 ATP
Phosphorylation and the redox reactions are ___, so the ratio of the number of NADH molecules to the number of ATP molecules is not a whole number
Not directly coupled with each other
The ATP yield varies slightly depending on ___ from the cytosol into the mitochondrion
the type of shuttle used to transport electrons
__ reduces the yield of ATP
The use of the proton-motive force generated by the redox reactions of respiration to drive other kinds of work
Breakdown of one glucose molecule
What effect would an absence of O2 have on the process shown in Figure 7.14?
Oxidative phosphorylation would eventually stop entirely, resulting in no ATP production by this process. Without oxygen to “pull” electrons down the electron transport chain, H+ would not be pumped into the mitochondrion’s intermembrane space and chemiosmosis would not occur.
In the absence of O2, as in question 1, what do you think would happen if you decreased the pH of the intermembrane space of the mitochondrion? Explain your answer.
Decreasing the pH means the addition of H+. This would establish a proton gradient even without the function of the electron transport chain, and we would expect ATP synthase to function and synthesize ATP. (In fact, it was experiments like this that provided support for chemiosmosis as an energy-coupling mechanism.)
Membranes must be fluid to function properly. How does the operation of the electron transport chain support that assertion?
One of the components of the electron transport chain, ubiquinone (Q), must be able to diffuse within the membrane. It could not do so if the membrane components were locked rigidly into place.
Briefly explain the mechanism by which ATP synthase produces ATP. List three locations in which ATP synthases are found.
The flow of H+ through the ATP synthase complex causes the rotor and attached rod to rotate, exposing catalytic sites in the knob portion that produce ATP from ADP and ℗i. ATP synthases are found in the inner mitochondrial membrane, the plasma membrane of prokaryotes, and membranes within chloroplasts.
Concept 7.5: Fermentation and anaerobic respiration enable cells to produce ATP without the use of oxygen
Glycolysis nets 2 ATP by substrate-level phosphorylation, whether oxygen is present or not. Under anaerobic conditions, either anaerobic respiration or fermentation can take place. In anaerobic respiration, an electron transport chain is present with a final electron acceptor other than oxygen. In fermentation, the electrons from NADH are passed to pyruvate or a derivative of pyruvate, regenerating the NAD+ required to oxidize more glucose. Two common types of fermentation are alcohol fermentation and lactic acid fermentation.
Fermentation, anaerobic respiration, and aerobic respiration all use glycolysis to oxidize glucose, but they differ in their final electron acceptor and whether an electron transport chain is used (respiration) or not (fermentation). Respiration yields more ATP; aerobic respiration, with O2 as the final electron acceptor, yields about 16 times as much ATP as does fermentation.
Glycolysis occurs in nearly all organisms and is thought to have evolved in ancient prokaryotes before there was O2 in the atmosphere.
Organisms that undergo anaerobic respiration have an electron transport chain but do not use ___
oxygen as a final electron acceptor at the end of the chain
Fermentation is a way of harvesting chemical energy without using either ___ or any _
oxygen; elcrotn transport chain
For aerobic respiration to continure, the cell must be supplied with oxygen to accept electrons. What is the elctron acceptor in fermentation?
Pyruvate
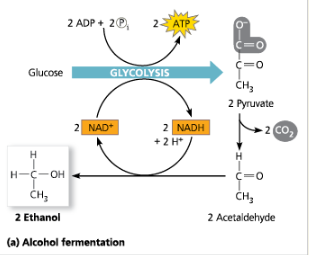
Alcohol Fermentation:
Glycolysis followed by teh reduction of pyruvate to ethyl alcohol, regenerating NAD+ and releasing carbon dioxide
Explain how alcohol fermentation starts with glucose and yeilds ethanol. Be sure to stress how NAD+ is recycled.
First carbon dioxide is released from pyruvate which forms acetaldehyde (C-C). Then, acetaldehyde is reduced by NADH. Now, the products are NAD+ and ethanol. This supplies the NAD+ needed for glycolysis.
Fermentation (a) Alcohol Fermentation
Fermentation (b) Lactic Acid Fermentation
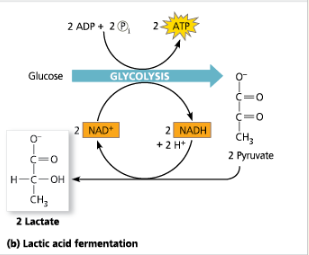
Lactic Acid Fermentation:
Glycolysis followed by the reduction of pyruvate to lactate, regenerating NAD+ with no release of carbon dioxide
Explain how lactic acid fermentation starts with glucose and yeilds ethanol. Be sure to stress how NAD+ is recycled.
NADH reduces pyruvate to lactate. Because NADH is oxidized to NAD+, the process fo glycolysis can start again
Lactate accumulates in muscles and is later converted to pyruvate by liver cells. This pyruvate can then complete ___
cellular respiration
__ use glycolysis to oxidize glucose and other organic fuels to pyruvate, with a net production of 2 ATP by substrate-level phosphorylation
Fermentation, anaerobic respiration, and aerobic respiration
In ___, NAD+ is the oxidizing agent that accepts electrons from food during glycolysis
Fermentation, anaerobic respiration, and aerobic respiration
How is NAD+ regnerated in different pathways?
In fermentation, pyruvate or acetaldehyde are the final electron acceptors while electrons carried by NADH are transferred to the ETC in cellular respiration. These both regenerate NAD+ for glycolysis from NADH.
Fermentation yields ___ molecules of ATP, produced by substrate-level phosphorylation
two
Cellular respiration harvests ___ energy from each sugar molecule than fermentation can
much more
Obligate Anaerobes:
An organism that only carries out fermentation or anaerobic respiration. Such organisms cnnot use oxygen and in fact may be poisoned by it
Facultative Anaerobes:
An organism that makes ATP by aerobic respiration if oxygen is present but that switches to anaerobic respiration or fermentation if oxygen is not present
Pyruvate as a Key Juncture in Catabolism
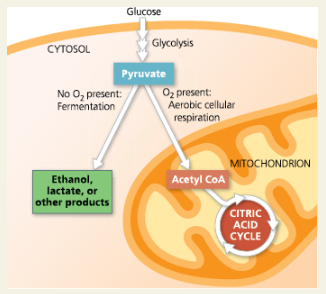
Explain why pyruvate is a key juncture in etabolism.
Pyruvate can undergo two different catabolic pathways: fermentation or aerobic cellular respiration. Between these paths, it can be catabolized into acetyl CoA, ethanol, lactate, or other products.
Consider the NADH formed during glycolysis. What is the final acceptor for its electrons during fermentation? What is the final acceptor for its electrons during aerobic respiration?
A derivative of pyruvate, such as acetaldehyde during alcohol fermentation, or pyruvate itself during lactic acid fermentation; oxygen during aerobic respiration
A glucose-fed yeast cell is moved from an aerobic environment to an anaerobic one. How would its rate of glucose consumption change if ATP were to be generated at the same rate?
The cell would need to consume glucose at a rate about 16 times the consumption rate in the aerobic environment (2 ATP are generated by fermentation versus up to 32 ATP by cellular respiration).
Which process yields more ATP, fermentation or anaerobic respiration? Explain.
Anaerobic respiration yields more ATP. The 2 ATP produced by substrate-level phosphorylation in glycolysis represent the total energy yield of fermentation. NADH passes its “high-energy” electrons to pyruvate or a derivative of pyruvate, recycling NAD+ and allowing glycolysis to continue. In anaerobic respiration, the NADH produced during glycolysis, as well as additional molecules of NADH produced as pyruvate is oxidized, are used to generate ATP molecules. An electron transport chain captures the energy of the electrons in NADH via a series of redox reactions; ultimately, the electrons are transferred to an electronegative molecule other than oxygen. Also, additional molecules of NADH are produced in anaerobic respiration as pyruvate is oxidized.
Concept 7.6: Glycolysis and the citric acid cycle connect to many other metabolic pathways
Catabolic pathways funnel electrons from many kinds of organic molecules into cellular respiration. Many carbohydrates can enter glycolysis, most often after conversion to glucose. Amino acids of proteins must be deaminated before being oxidized. The fatty acids of fats undergo beta oxidation to two-carbon fragments and then enter the citric acid cycle as acetyl CoA. Anabolic pathways can use small molecules from food directly or build other substances using intermediates of glycolysis or the citric acid cycle.
The catabolism of various molecuels from food
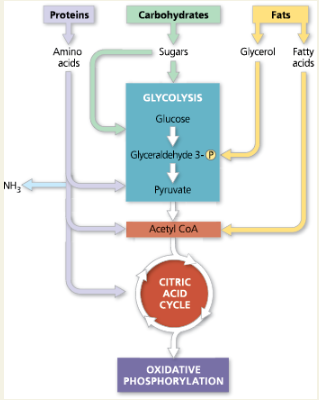
What three organic molecules are often used to make ATP by cellular respiration?
Carbohydrates, fats, and proteins
Deamination:
Removing an amino group (from amino acids so they can feed into glycolysis or the citric acid cycle)
Explain the difference in energy usage between the catabolic reactions of cellular respiration and anabolic pathways of biosynthesis
Cellular respiration breaks down glucose catabolically resulting in energy while biosynthesis uses energy to anabolically build carbon skeletons in cells.
Explain how AMP stimulates cellular respiration while citrate and ATP inhibit it
AMP is an allosteric activator and binds to (PFK) enzymes in their active form during cellular respiration. Citrate binds to PFK inhibiting it, and ATP is also an allosteric inhibitor.
Beta Oxidation:
A metabolic sequence that breaks fatty acids down to two-carbon fragments that enter the citric acid cycle as acetyl CoA
In addition to calories, food must also provide ___
In addition to calories, food must also provide the carbon skeletons that cells require to make their own molecules
The energy that keeps us alive is ___ by cellular respiration. We are tapping energy that was stored in food by photosynthesis
released, not produced
Compare the structure of a fat (see Figure 3.13) with that of a carbohydrate (see Figure 3.8). What features of their structures make fat a much better fuel?
The fat is much more reduced; it has many “CH2” units, and in all these bonds the electrons are equally shared. The electrons present in a carbohydrate molecule are already somewhat oxidized (shared unequally in bonds), as quite a few of them are bound to oxygen. Electrons that are equally shared, as in fat, have a higher energy level than electrons that are unequally shared, as in carbohydrates. Thus, fats are much better fuels than carbohydrates.
When might your body synthesize fat molecules?
When you consume more food than necessary for metabolic processes, your body synthesizes fat as a way of storing energy for later use.
During intense exercise, can a muscle cell use fat as a concentrated source of chemical energy? Explain.
When oxygen is present, the fatty acid chains containing most of the energy of a fat are oxidized and fed into the citric acid cycle and the electron transport chain. During intense exercise, however, oxygen is scarce in muscle cells, so ATP must be generated by glycolysis alone. A very small part of the fat molecule, the glycerol backbone, can be oxidized via glycolysis, but the amount of energy released by this portion is insignificant compared with that released by the fatty acid chains. (This is why moderate exercise, staying below 70% maximum heart rate, is better for burning fat—because enough oxygen remains available to the muscles.)
Describe how the catabolic pathways of glycolysis and the citric acid cycle intersect with anabolic pathways in the metabolism of a cell.
The ATP produced by catabolic pathways is used to drive anabolic pathways. Also, many of the intermediates of glycolysis and the citric acid cycle are used in the biosynthesis of a cell’s molecules.
AP BIO CHAPTER 7: Cellular Respiration and Fermentation
Introduction
7.1 Catabolic pathways yield energy by oxidizing organic fuels
7.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate
7.3 After pyruvate is oxidized, the citric acid cycle completes the energy-yielding oxidation of organic molecules
7.4 During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis
7.5 Fermentation and anaerobic respiration enable cells to produce ATP without the use of oxygen
7.6 Glycolysis and the citric acid cycle connect to many other metabolic pathways
BIG IDEAS: Heterotrophic organisms must capture free energy from their environment to maintain life (Big Idea 2). Common to all are core processes that move energy and matter through living systems and thereby support life (Big Ideas 1 & 4).
Photosynthesis vs Respiration
Photosynthesis generates oxygen and organic molecules. Respiration breaks down this fuel.
Energy flow in an ecosystem

Key Pathways:
Glycolysis
Pyruvate Oxidation
Citric Acid Cycle
Oxidative Phosphorylation
Fermentation
Concept 7.1: Catabolic pathways yield energy by oxidizing organic fuels
Cells break down glucose and other organic fuels to yield chemical energy in the form of ATP. Fermentation is a process that results in the partial degradation of glucose without the use of oxygen. Cellular respiration is a more complete breakdown of glucose; in aerobic respiration, oxygen is used as a reactant. The cell taps the energy stored in food molecules through redox reactions**, in which one substance partially or totally shifts electrons to another.** Oxidation is the loss of electrons from one substance, while reduction is the addition of electrons to the other.
During aerobic respiration, glucose (C6H12O6) is oxidized to CO2, and O2 is reduced to H2O. Electrons lose potential energy during their transfer from glucose or other organic compounds to oxygen. Electrons are usually passed first to NAD+, reducing it to NADH, and then from NADH to an electron transport chain, which conducts them to O2 in energy-releasing steps. The energy is used to make ATP.
Aerobic respiration occurs in three stages: (1) glycolysis, (2) pyruvate oxidation and the citric acid cycle, and (3) oxidative phosphorylation (electron transport and chemiosmosis).
What plays a major role in catabolic pathways?
Electron transfer
Cells degrade organic molecules with high potential energy →
energy to do work, some dissipated as heat
Fermentation:
A catabolic process that makes a limited amount of ATP from glucose (or other organic molecules) without an electron transport chain or the use of oxygen and that produces a characteristic end product, such as ethyl alcohol or lactic acid
Aerobic Respiration:
A catabolic pathway for organic molecules, using O2 as the final electron acceptor in an electron transport chain and ultimately producing ATP. (Oxygen and organic fuel consumed as reactant)
Is aerobic respiration efficient, and where is it carried out?
It is the most efficient catabolic pathway and is carried out in most eukaryotic cells and many prokaryotic organisms.
Anaerobic Respiration:
Some prokaryotes use substances other than oxygen as reactants in a similar process that harvests chemical energy without oxygen (the prefix an- means “without”).
Cellular Respiration:
The catabolic pathways of aerobic and anaerobic respiration break down organic molecules and use an electron transport chain for the production of ATP.
_____ is often used to refer to the aerobic process
cellular respiration
Oxidation-Reduction Reactions / Redox Reactions:
A chemical reaction involving the complete or partial transfer of one or more electrons from one reactant to another
Oxidation:
The complete or partial loss of electrons from a substance involved in a redox reaction
Reduction:
The complete or partial addition of electrons to a substance involved in a redox reaction (Note that adding electrons is called reduction; adding negatively charged electrons to an atom reduces the amount of positive charge of that atom.)
Redox Reaction

Reducing Agent:
The electron donor in a redox reaction
Oxidizing Agent:
The electron acceptor in a redox reaction
Why would a reaction release energy to the surroundings?
The electrons lose potential energy when they end up being shared unequally, spending more time near electronegative atoms such as oxygen
Methane Combustion

Because oxygen is so ____, it is one of the most powerful of all oxidizing agents.
electronegative
____ must be added to pull an electron away from an atom.
energy
Electronegativity:
How an atom pulls on electrons (higher electronegativity = more energy requires to take an electron away from it)
As in the combustion of methane or gasoline…
The fuel (glucose) is oxidized and oxygen is reduced. The electrons lose potential energy along the way, and energy is released.
Why are organic molecules with an abundance of hydrogen excellent fuels?
Their bonds are a source of “hilltop” electrons, whose energy may be released as these electrons “fall” down an energy gradient when they are transferred to oxygen
Only ___ holds back the flood of electrons to a lower energy state.
the barrier of activation energy
NAD+ (Nicotinamide Adenine Dinucleotide):
A coenzyme that cycles easily between oxidized and reduced (NAD+ and NADH) states, thus acting as an electron carrier
How does NAD+ trap electrons from glucose and other organic molecules in food?
Enzymes called dehydrogenases remove a pair of hydrogen atoms (2 electrons and 2 protons) from the substrate (glucose, in this example), thereby oxidizing it. The enzyme delivers the 2 electrons along with 1 proton to its coenzyme, NAD+, forming NADH (Figure 7.4). The other proton is released as a hydrogen ion (H+) into the surrounding solution
Nicotinamide (oxidized form)

Nicotinamide (reduced form)

Each NADH molecule formed during respiration represents ___ that can be tapped to make ATP when the electrons complete their “fall” down an energy gradient
stored energy
In cellular respiration, the hydrogen that reacts with oxygen is derived from ___ rather than H2
organic molecules
Instead of occurring in one explosive reaction, respiration uses an electron transport chain to break the fall of electrons to oxygen into ___
several energy-releasing steps
Electron Transport Chain:
A sequence of electron carrier molecules (membrane proteins) that shuttle electrons down a series of redox reactions that release energy used to make ATP
Where are electron transport chains?
They are in the inner membrane of the mitochondria of eukaryotic cells and the plasma membrane of aerobically respiring prokaryotes
Electrons removed from glucose are shuttled by NADH to the ___ end of the chain. At the _ end, O2 captures these electrons along with hydrogen nuclei (H+), forming water.
top/higher-energy; bottom/lower-energy
An explosion
The one-step exergonic reaction of hydrogen with oxygen to form water releases a large amount of energy in the form of heat and light
Uncontrolled Reaction

Cellular Respiration

Electron transfer from NADH to oxygen is an ___ reaction
exergonic
Each “downhill” carrier is more ___ than and thus capable of oxidizing, its “uphill” neighbor, with oxygen at the bottom of the chain.
electronegative
Downhill Route
glucose → NADH → electron transport chain → oxygen
The Stages of Cellular Respiration: A Preview
Glycolysis:
A series of reactions that ultimately splits glucose into pyruvate, Glycolysis occurs in almost all living cells, serving as the starting point for fermentation or cellular respiration.
Where does glycolysis occur?
It occurs in the cytosol
___ begins the degradation process by breaking glucose into two molecules of a compound called pyruvate
Glycolysis
Citric Acid Cycle (Krebs Cycle):
A chemical cycle involving eight steps that completes the metabolic breakdown of glucose molecules begun in glycolysis by oxidizing acetyl CoA (derived from pyruvate) to carbon dioxide
Where does the citric acid cycle occur?
It occurs in the mitochondrion in eukaryotic cells and in the cytosol of prokaryotes.
The citric acid cycle with ___ is the second major stage in cellular respiration
pyruvate oxidation
In eukaryotes, ___ enters the mitochondrion and is oxidized to a compound called acetyl CoA, which enters the citric acid cycle
pyruvate
When compounds lose electrons, they ___ energy; when compounds gain electrons, they _ energy.
lose; gain
Show the normal, downhill route most electrons follow in cellular respiration.
Glucose → NADH → ETC → Oxygen
Figure 7.6 An overview of cellular respiration

During oxidative phosphorylation, electron transport chains convert the chemical energy to a form used for ATP synthesis in the process called ___
chemiosmosis
Some of the steps of glycolysis and the citric acid cycle are redox reactions in which dehydrogenases transfer electrons from substrates to NAD+, forming ___
NADH
In the third stage of respiration, the electron transport chain ___ (most often via NADH) from the breakdown products of the first two stages and passes these electrons from one molecule to another. At the end of the chain, the electrons are combined with molecular oxygen and hydrogen ions (H+), forming water (see Figure 7.5b).
accepts electrons
The energy released at each step of the chain is stored in a form that the mitochondrion (or prokaryotic cell) and can be used to make ___
ATP from ADP
Oxidative Phosphorylation:
The production of ATP using energy derived from the redox reactions of an electron transport chain; the third major stage of cellular respiration
In eukaryotic cells, ___ is the site of electron transport and chemiosmosis, the processes that together constitute oxidative phosphorylation. (In prokaryotes, these processes take place in the plasma membrane.)
the inner membrane of the mitochondrion
Substrate-Level Phosphorylation:
The enzyme-catalyzed formation of ATP by direct transfer of a phosphate group to ADP from an intermediate substrate in catabolism
Figure 7.7 Substrate-Level Phosphorylation

Review Figure 6.8. Do you think the potential energy is higher for the reactants or the products in the reaction shown above? Explain.
Because there is no external source of energy for the reaction, it must be exergonic, and the reactants must be at a higher energy level than the products.
Compare and contrast aerobic and anaerobic respiration.
Both processes include glycolysis, the citric acid cycle, and oxidative phosphorylation. In aerobic respiration, the final electron acceptor is molecular oxygen (O2); in anaerobic respiration, the final electron acceptor is a different substance.
Name and describe the two ways in which ATP is made during cellular respiration. During what stage(s) in the process does each type occur?
Substrate-level phosphorylation, which occurs during glycolysis and the citric acid cycle, involves the direct transfer of a phosphate group from an organic substrate to ADP by an enzyme. The process of oxidative phosphorylation occurs during the third stage of cellular respiration, which is called oxidative phosphorylation. In this process, the synthesis of ATP from ADP and inorganic phosphate (℗i) is powered by the redox reactions of the electron transport chain.
WHAT IF? If the following redox reaction occurred, which compound would be oxidized? Which reduced? C4H6O5 + NAD+ → C4H4O5 + NADH + H+
C4H6O5 would be oxidized, and NAD+ would be reduced.
Describe the difference between the two processes in cellular respiration that produce ATP: oxidative phosphorylation and substrate-level phosphorylation.
Most of the ATP produced in cellular respiration comes from oxidative phosphorylation, in which the energy released from redox reactions in an electron transport chain is used to produce ATP. In substrate-level phosphorylation, an enzyme directly transfers a phosphate group to ADP from an intermediate substrate. All ATP production in glycolysis occurs by substrate-level phosphorylation; this form of ATP production also occurs at one step in the citric acid cycle.
Concept 7.2: Glycolysis harvests chemical energy by oxidizing glucose to pyruvate
Glycolysis (“splitting of sugar”) is a series of reactions that breaks down glucose into two pyruvate molecules, which may go on to enter the citric acid cycle, and nets 2 ATP and 2 NADH per glucose molecule.
What does glycolysis mean?
“sugar splitting“
The starting product of glycolysis is the six-carbon sugar ___, and the ending product is two three-carbon compounds termed _
glucose; pyruvate
What is the net energy yield from glycolysis, per glucose molecule?
2 ATP plus 2 NADH
Energy Investment Phase

Energy Payoff Phase

Net

First 5 Steps of Glycolysis

Last 5 Steps of Glycolysis

What would happen if you removed the dihydroxyacetone phosphate generated in step 4 as fast as it was produced?
The removal would probably stop glycolysis, or at least slow it down, since it would push the equilibrium for step 5 toward the bottom (toward DHAP). If less (or no) glyceraldehyde 3-phosphate were available, step 6 would slow down (or be unable to occur).
What two stages use ATP in glycolysis?
Stages #1 and #3
What stage forms ATP in glycolysis?
Stage #7
What stage forms NADH in glycolysis?
Stage #6
If ___ is present, the chemical energy stored in pyruvate and NADH can be extracted by pyruvate oxidation, the citric acid cycle, and oxidative phosphorylation
O2
Glycolysis occurs in the ___ of the cell
cytosol
During step 6 in Figure 7.9, which molecule acts as the oxidizing agent? The reducing agent?
NAD+ acts as the oxidizing agent in step 6, accepting electrons from glyceraldehyde 3-phosphate (G3P), which thus acts as the reducing agent.
Which reactions are the source of energy for the formation of ATP and NADH in glycolysis?
The oxidation of the three-carbon sugar glyceraldehyde 3-phosphate yields energy. In this oxidation, electrons and H+ are transferred to NAD+, forming NADH, and a phosphate group is attached to the oxidized substrate. ATP is then formed by substrate-level phosphorylation when this phosphate group is transferred to ADP.
Concept 7.3: After pyruvate is oxidized, the citric acid cycle completes the energy-yielding oxidation of organic molecules
In eukaryotic cells, pyruvate enters the mitochondrion and is oxidized to acetyl CoA, which is further oxidized in the citric acid cycle
Acetyl CoA:
Acetyl coenzyme A: the entry compound for the citric acid cycle in cellular respiration, formed from a two-carbon fragment of pyruvate attached to a coenzyme
Overview of pyruvate oxidation and the citric acid cycle

A closer look at the citric acid cycle

To enter the citric acid cycle, pyruvate must enter the mitochondria by active transport. Three things are necessary to convert pyruvate to acetyl CoA. Explain the 3 steps.
Carbon is taken out and later released and CO2, NAD+ turns into NADH, and CoA joins C-C to make acetyl
How many times does the citric acid cycle occur for each molecule of glucose?
Twice, once per acetyl CoA
Referring to Figure 7.11, how many NADHs are formed?
3
How many carbons are lost as pyruvate is oxidized?
2
What are the carbons released as?
Carbon dioxide
How many FADH2 have been formed?
1
How many ATPs have been formed?
1
How do you find the number of molecules formed from a single glucose?
Since glucose requires two cycles, double the amount of molecules formed from one cycle
The step that converts pyruvate to acetyl CoA at the top of the diagram also occurs twice per glucose. This step accounts for two additional reduced ___ molecules and two carbon dioxide molecules.
NADH
Name the molecules that conserve most of the energy from redox reactions of the citric acid cycle. How is this energy converted to a form that can be used to make ATP?
NADH and FADH2; they will donate electrons to the electron transport chain
What processes in your cells produce the CO2 that you exhale?
CO2 is released from the pyruvate that is the end product of glycolysis, and CO2 is also released during the citric acid cycle.
What molecular products indicate the complete oxidation of glucose during cellular respiration?
The release of six molecules of CO2 represents the complete oxidation of glucose. During the processing of two pyruvates to acetyl CoA, the fully oxidized carboxyl groups (—COO–) are given off as 2 CO2. The remaining four carbons are released as CO2 in the citric acid cycle as citrate is oxidized back to oxaloacetate.
Concept 7.4: During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis
NADH and FADH2 transfer electrons to the electron transport chain. Electrons move down the chain, losing energy in several energy-releasing steps. Finally, electrons are passed to O2, reducing it to H2O.
Along the electron transport chain, electron transfer causes protein complexes to move H+ from the mitochondrial matrix (in eukaryotes) to the intermembrane space, storing energy as a proton-motive force (H+ gradient). As H+ diffuses back into the matrix through ATP synthase, its passage drives the phosphorylation of ADP, an energy-coupling mechanism called chemiosmosis.
About 34% of the energy stored in a glucose molecule is transferred to ATP during cellular respiration, producing a maximum of about 32 ATP.
Prosthetic Groups:
Nonprotein components essential for the catalytic functions of certain enzymes; they are tightly bound to ETC proteins
Electron carriers ___ between reduced and oxidized states as they accept and then donate electrons
alternate
Each component of the chain becomes ___ when it accepts electrons from its “uphill” neighbor, which has a lower affinity for electrons (is less electronegative). It then returns to its _ form as it passes electrons to its “downhill,” more electronegative neighbor.
reduced; oxidized
Free-energy change during electron transport

Each member of the electron transport chain is lower in ___ than the preceding member of the chain but higher in _.
free energy; electronegativity
The molecule at zero free energy, which is ___, is the lowest of all the molecules in free energy and highest in electronegativity.
O2
Oxygen stabilizes the electrons by combining with ___ to form _
two hydrogen ions, H2O
The two electron carriers molecules that feed electrons into the electron transport system are ___
NADH and FADH2
Cytochromes:
An iron-containing protein that is a component of electron transport chains in the mitochondria and chloroplasts of eukaryotic cells and the plasma membranes of prokaryotic cells
Heme Group:
A cytochrome’s prosthetic group; has an iron atom that accepts and donates electrons
The last cytochrome of the chain, Cyt a3, passes its electrons to oxygen, which is ___ electronegative
very
Although NADH and FADH2 each donate an equivalent number of electrons (2) for oxygen reduction, the electron transport chain provides about one-third less energy for ATP synthesis when the electron donor is ___
FADH2 rather than NADH
ATP Synthase:
A complex of several membrane proteins that functions in chemiosmosis with adjacent electron transport chains, using the energy of a hydrogen ion (proton) concentration gradient to make ATP.
Where are ATP synthases found?
ATP synthases are found in the inner mitochondrial membranes of eukaryotic cells and in the plasma membranes of prokaryotes.
Chemiosmosis:
An energy-coupling mechanism that uses energy stored in the form of a hydrogen ion gradient across a membrane to drive cellular work, such as the synthesis of ATP.
Under aerobic conditions, most ATP synthesis in cells occurs by ___
chemiosmosis
ATP synthase, a molecular mill: (a) The ATP synthase protein complex functions as a mill, powered by the flow of hydrogen ions.

ATP synthase, a molecular mill: (b) This computer model shows the four parts of ATP synthase. Each part consists of a number of polypeptide subunits. The entire structure of the gray region has not yet been determined and is an area of active research.

Label the rotor, stator, internal rod, and catalytic knob in part (b), the computer model.

Explain the overall concept of how ATP synthase uses the flow of H+ ions to produce ATP
H+ ions flow down their gradient which turns a rod like a mill. This activates catalytic sites that produce ATP from ADP and Pi.
Chemiosmosis couples the electron transport chain to ATP synthesis

What is the role of the ETC in forming the H+ gradient across the inner mitochondrial membrane?
As NADH and FADH2 deposit electrons to the ETC, NAD+ and FAD+ are formed, and H+ ions are pumped to the inner membrane space. This creates a gradient for H+ to flow back through the ATP synthase
How do chemiosmosis and proton-motive force relate to oxidative phosphorylation?
ATP synthesis is powered by chemiosmosis, using an H+ gradient to drive work. They flow with the proton-motive force, potential energy in the H+ gradient.
If complex IV were nonfunctional, could chemiosmosis produce any ATP, and if so, how would the rate of synthesis differ?
At first, some ATP could be made, since electron transport could proceed as far as complex III, and a small H+ gradient could be built up. Soon, however, no more electrons could be passed to complex III because it could not be reoxidized by passing its electrons to complex IV.
Proton-Motive Force:
The potential energy stored in the form of a proton electrochemical gradient generated by the pumping of hydrogen ions (H+) across a biological membrane during chemiosmosis
Chemiosmosis is an energy-coupling mechanism that uses energy stored in the form of an ___ to drive cellular work
H+gradient across a membrane
What sequence does most energy flow in during respiration?
glucose → NADH → electron transport chain → proton-motive force → ATP
ATP yield per molecule of glucose at each stage of cellular respiration

Explain exactly how the total of 26 or 28 ATP (see the yellow bar) was calculated
First, there are 2 NADH from the oxidation of pyruvate plus 6 NADH from the citric acid cycle (CAC); 8 NADH × 2.5 ATP/NADH = 20 ATP. Second, there are 2 FADH2 from the CAC; 2 FADH2 × 1.5 ATP/FADH2 = 3 ATP. Third, the 2 NADH from glycolysis enter the mitochondrion through one of two types of shuttles. They pass their electrons either to 2 FAD, which becomes FADH2 and results in 3 ATP or 2 NAD+, which becomes NADH and results in 5 ATP. Thus, 20 + 3 + 3 = 26 ATP or 20 + 3 + 5 = 28 ATP from all NADH and FADH2.
How many maximum molecules of ATP are formed from NADH and FADH2?
1 NADH results in 10 H+ being pumped, and it takes about 4 H+ for one ATP. That means 1 NADH results in about 2.5 ATP molecules. Since FADH2’s electrons enter the chain later, it only transfers enough H+ to the inner membrane space for about 1.5 ATP.
List the products in glycolysis
2 NADH, 2 Pyruvate, 2 ATP
List the products in pyruvate oxidation
2 Acetyl CoA, 2 NADH, 2 CO2
List the products in the citric acid cycle per one glucose/two turns
4 CO2, 6 NADH, 2 ATP, 2 FADH2
Why is the total count about 36 or 38 ATP molecules rather than a specific number?
The 2 NADH from glycolysis enter the mitochondrion through one of two types of shuttles. They pass their electrons either to 2 FAD, which becomes FADH2 and results in 3 ATP or 2 NAD+ which becomes NADH and results in 5 ATP
Phosphorylation and the redox reactions are ___, so the ratio of the number of NADH molecules to the number of ATP molecules is not a whole number
Not directly coupled with each other
The ATP yield varies slightly depending on ___ from the cytosol into the mitochondrion
the type of shuttle used to transport electrons
__ reduces the yield of ATP
The use of the proton-motive force generated by the redox reactions of respiration to drive other kinds of work
Breakdown of one glucose molecule

What effect would an absence of O2 have on the process shown in Figure 7.14?
Oxidative phosphorylation would eventually stop entirely, resulting in no ATP production by this process. Without oxygen to “pull” electrons down the electron transport chain, H+ would not be pumped into the mitochondrion’s intermembrane space and chemiosmosis would not occur.
In the absence of O2, as in question 1, what do you think would happen if you decreased the pH of the intermembrane space of the mitochondrion? Explain your answer.
Decreasing the pH means the addition of H+. This would establish a proton gradient even without the function of the electron transport chain, and we would expect ATP synthase to function and synthesize ATP. (In fact, it was experiments like this that provided support for chemiosmosis as an energy-coupling mechanism.)
Membranes must be fluid to function properly. How does the operation of the electron transport chain support that assertion?
One of the components of the electron transport chain, ubiquinone (Q), must be able to diffuse within the membrane. It could not do so if the membrane components were locked rigidly into place.
Briefly explain the mechanism by which ATP synthase produces ATP. List three locations in which ATP synthases are found.
The flow of H+ through the ATP synthase complex causes the rotor and attached rod to rotate, exposing catalytic sites in the knob portion that produce ATP from ADP and ℗i. ATP synthases are found in the inner mitochondrial membrane, the plasma membrane of prokaryotes, and membranes within chloroplasts.
Concept 7.5: Fermentation and anaerobic respiration enable cells to produce ATP without the use of oxygen
Glycolysis nets 2 ATP by substrate-level phosphorylation, whether oxygen is present or not. Under anaerobic conditions, either anaerobic respiration or fermentation can take place. In anaerobic respiration, an electron transport chain is present with a final electron acceptor other than oxygen. In fermentation, the electrons from NADH are passed to pyruvate or a derivative of pyruvate, regenerating the NAD+ required to oxidize more glucose. Two common types of fermentation are alcohol fermentation and lactic acid fermentation.
Fermentation, anaerobic respiration, and aerobic respiration all use glycolysis to oxidize glucose, but they differ in their final electron acceptor and whether an electron transport chain is used (respiration) or not (fermentation). Respiration yields more ATP; aerobic respiration, with O2 as the final electron acceptor, yields about 16 times as much ATP as does fermentation.
Glycolysis occurs in nearly all organisms and is thought to have evolved in ancient prokaryotes before there was O2 in the atmosphere.
Organisms that undergo anaerobic respiration have an electron transport chain but do not use ___
oxygen as a final electron acceptor at the end of the chain
Fermentation is a way of harvesting chemical energy without using either ___ or any _
oxygen; elcrotn transport chain
For aerobic respiration to continure, the cell must be supplied with oxygen to accept electrons. What is the elctron acceptor in fermentation?
Pyruvate
Alcohol Fermentation:
Glycolysis followed by teh reduction of pyruvate to ethyl alcohol, regenerating NAD+ and releasing carbon dioxide
Explain how alcohol fermentation starts with glucose and yeilds ethanol. Be sure to stress how NAD+ is recycled.
First carbon dioxide is released from pyruvate which forms acetaldehyde (C-C). Then, acetaldehyde is reduced by NADH. Now, the products are NAD+ and ethanol. This supplies the NAD+ needed for glycolysis.
Fermentation (a) Alcohol Fermentation

Fermentation (b) Lactic Acid Fermentation

Lactic Acid Fermentation:
Glycolysis followed by the reduction of pyruvate to lactate, regenerating NAD+ with no release of carbon dioxide
Explain how lactic acid fermentation starts with glucose and yeilds ethanol. Be sure to stress how NAD+ is recycled.
NADH reduces pyruvate to lactate. Because NADH is oxidized to NAD+, the process fo glycolysis can start again
Lactate accumulates in muscles and is later converted to pyruvate by liver cells. This pyruvate can then complete ___
cellular respiration
__ use glycolysis to oxidize glucose and other organic fuels to pyruvate, with a net production of 2 ATP by substrate-level phosphorylation
Fermentation, anaerobic respiration, and aerobic respiration
In ___, NAD+ is the oxidizing agent that accepts electrons from food during glycolysis
Fermentation, anaerobic respiration, and aerobic respiration
How is NAD+ regnerated in different pathways?
In fermentation, pyruvate or acetaldehyde are the final electron acceptors while electrons carried by NADH are transferred to the ETC in cellular respiration. These both regenerate NAD+ for glycolysis from NADH.
Fermentation yields ___ molecules of ATP, produced by substrate-level phosphorylation
two
Cellular respiration harvests ___ energy from each sugar molecule than fermentation can
much more
Obligate Anaerobes:
An organism that only carries out fermentation or anaerobic respiration. Such organisms cnnot use oxygen and in fact may be poisoned by it
Facultative Anaerobes:
An organism that makes ATP by aerobic respiration if oxygen is present but that switches to anaerobic respiration or fermentation if oxygen is not present
Pyruvate as a Key Juncture in Catabolism

Explain why pyruvate is a key juncture in etabolism.
Pyruvate can undergo two different catabolic pathways: fermentation or aerobic cellular respiration. Between these paths, it can be catabolized into acetyl CoA, ethanol, lactate, or other products.
Consider the NADH formed during glycolysis. What is the final acceptor for its electrons during fermentation? What is the final acceptor for its electrons during aerobic respiration?
A derivative of pyruvate, such as acetaldehyde during alcohol fermentation, or pyruvate itself during lactic acid fermentation; oxygen during aerobic respiration
A glucose-fed yeast cell is moved from an aerobic environment to an anaerobic one. How would its rate of glucose consumption change if ATP were to be generated at the same rate?
The cell would need to consume glucose at a rate about 16 times the consumption rate in the aerobic environment (2 ATP are generated by fermentation versus up to 32 ATP by cellular respiration).
Which process yields more ATP, fermentation or anaerobic respiration? Explain.
Anaerobic respiration yields more ATP. The 2 ATP produced by substrate-level phosphorylation in glycolysis represent the total energy yield of fermentation. NADH passes its “high-energy” electrons to pyruvate or a derivative of pyruvate, recycling NAD+ and allowing glycolysis to continue. In anaerobic respiration, the NADH produced during glycolysis, as well as additional molecules of NADH produced as pyruvate is oxidized, are used to generate ATP molecules. An electron transport chain captures the energy of the electrons in NADH via a series of redox reactions; ultimately, the electrons are transferred to an electronegative molecule other than oxygen. Also, additional molecules of NADH are produced in anaerobic respiration as pyruvate is oxidized.
Concept 7.6: Glycolysis and the citric acid cycle connect to many other metabolic pathways
Catabolic pathways funnel electrons from many kinds of organic molecules into cellular respiration. Many carbohydrates can enter glycolysis, most often after conversion to glucose. Amino acids of proteins must be deaminated before being oxidized. The fatty acids of fats undergo beta oxidation to two-carbon fragments and then enter the citric acid cycle as acetyl CoA. Anabolic pathways can use small molecules from food directly or build other substances using intermediates of glycolysis or the citric acid cycle.
The catabolism of various molecuels from food

What three organic molecules are often used to make ATP by cellular respiration?
Carbohydrates, fats, and proteins
Deamination:
Removing an amino group (from amino acids so they can feed into glycolysis or the citric acid cycle)
Explain the difference in energy usage between the catabolic reactions of cellular respiration and anabolic pathways of biosynthesis
Cellular respiration breaks down glucose catabolically resulting in energy while biosynthesis uses energy to anabolically build carbon skeletons in cells.
Explain how AMP stimulates cellular respiration while citrate and ATP inhibit it
AMP is an allosteric activator and binds to (PFK) enzymes in their active form during cellular respiration. Citrate binds to PFK inhibiting it, and ATP is also an allosteric inhibitor.
Beta Oxidation:
A metabolic sequence that breaks fatty acids down to two-carbon fragments that enter the citric acid cycle as acetyl CoA
In addition to calories, food must also provide ___
In addition to calories, food must also provide the carbon skeletons that cells require to make their own molecules
The energy that keeps us alive is ___ by cellular respiration. We are tapping energy that was stored in food by photosynthesis
released, not produced
Compare the structure of a fat (see Figure 3.13) with that of a carbohydrate (see Figure 3.8). What features of their structures make fat a much better fuel?
The fat is much more reduced; it has many “CH2” units, and in all these bonds the electrons are equally shared. The electrons present in a carbohydrate molecule are already somewhat oxidized (shared unequally in bonds), as quite a few of them are bound to oxygen. Electrons that are equally shared, as in fat, have a higher energy level than electrons that are unequally shared, as in carbohydrates. Thus, fats are much better fuels than carbohydrates.
When might your body synthesize fat molecules?
When you consume more food than necessary for metabolic processes, your body synthesizes fat as a way of storing energy for later use.
During intense exercise, can a muscle cell use fat as a concentrated source of chemical energy? Explain.
When oxygen is present, the fatty acid chains containing most of the energy of a fat are oxidized and fed into the citric acid cycle and the electron transport chain. During intense exercise, however, oxygen is scarce in muscle cells, so ATP must be generated by glycolysis alone. A very small part of the fat molecule, the glycerol backbone, can be oxidized via glycolysis, but the amount of energy released by this portion is insignificant compared with that released by the fatty acid chains. (This is why moderate exercise, staying below 70% maximum heart rate, is better for burning fat—because enough oxygen remains available to the muscles.)
Describe how the catabolic pathways of glycolysis and the citric acid cycle intersect with anabolic pathways in the metabolism of a cell.
The ATP produced by catabolic pathways is used to drive anabolic pathways. Also, many of the intermediates of glycolysis and the citric acid cycle are used in the biosynthesis of a cell’s molecules.
 Knowt
Knowt