
Properties of Matter!
Properties of Matter!
What is matter?
Matter is anything that occupies space and has mass. If you can see, touch, taste, smell, or feel it then it's matter.
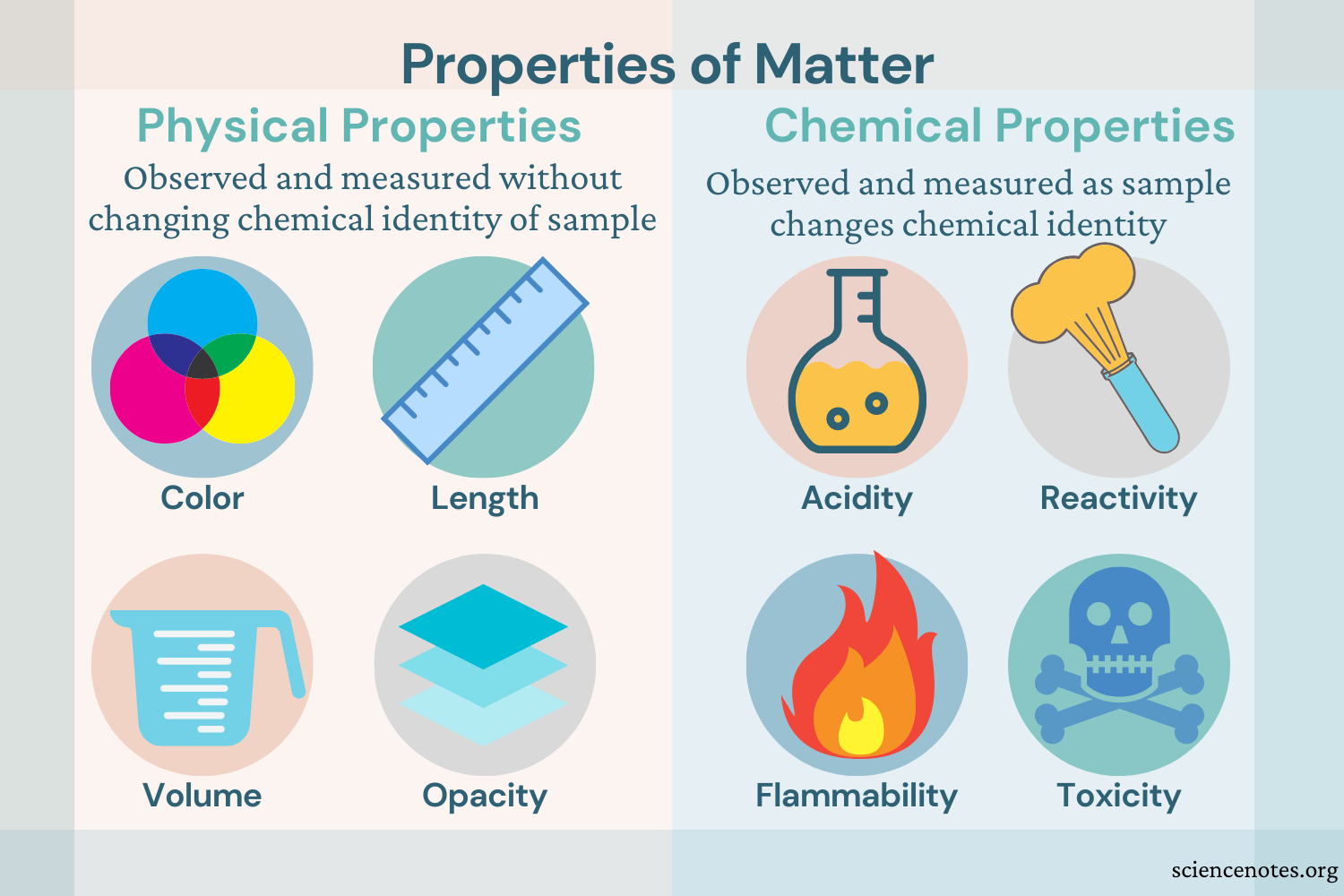
A property describes how an object looks, feels, or acts. All properties of matter are classified as either physical or chemical.
Physical Property - is usually observed with our senses
Examples...
Color - the quality of an object or substance with respect to the reflection of light
Size - an object's overall dimensions
Volume - the amount of space a substance or object occupies
Density - the ratio of mass and volume in a substance
Boiling Point/Melting Point - the temperature at which something boils or melts
Magnetism - whether or not something is magnetic
Solubility - how easily something dissolves in another substance
Physical properties are broken down into two different categories...
Intensive - they do not depend on the amount of the substance present; ex. the density of a substance at room temperature is the same no matter how much of the substance that you have
Other examples of intensive properties: color, odor, temperature, freezing point, melting point, boiling point, density, state of matter, malleability, and ductility
Extensive - they depend on the amount of matter being measured; ex. mass, length, and volume measures depending on how much of the object that you have
Other examples of extensive properties: size, length, width, volume, mass, and weight
Extensive properties can be added together. For example, two pennies will have more mass than one penny because the mass of two is greater than the mass of one.

Chemical Property - any characteristic that can be determined only by changing a substance's identity, possibly through a chemical reaction
Examples...
Reactivity - the likelihood of a substance to undergo a chemical reaction
Toxicity - how poisonous or damaging a chemical substance may be to organisms
Flammability - whether a substance will burn when exposed to a flame
Combustibility - the measure of how easily a substance will burn in oxygen
When determining the properties of matter, if you can identify the properties of the substance without changing it, then it is a physical property. If not, it is a chemical property.
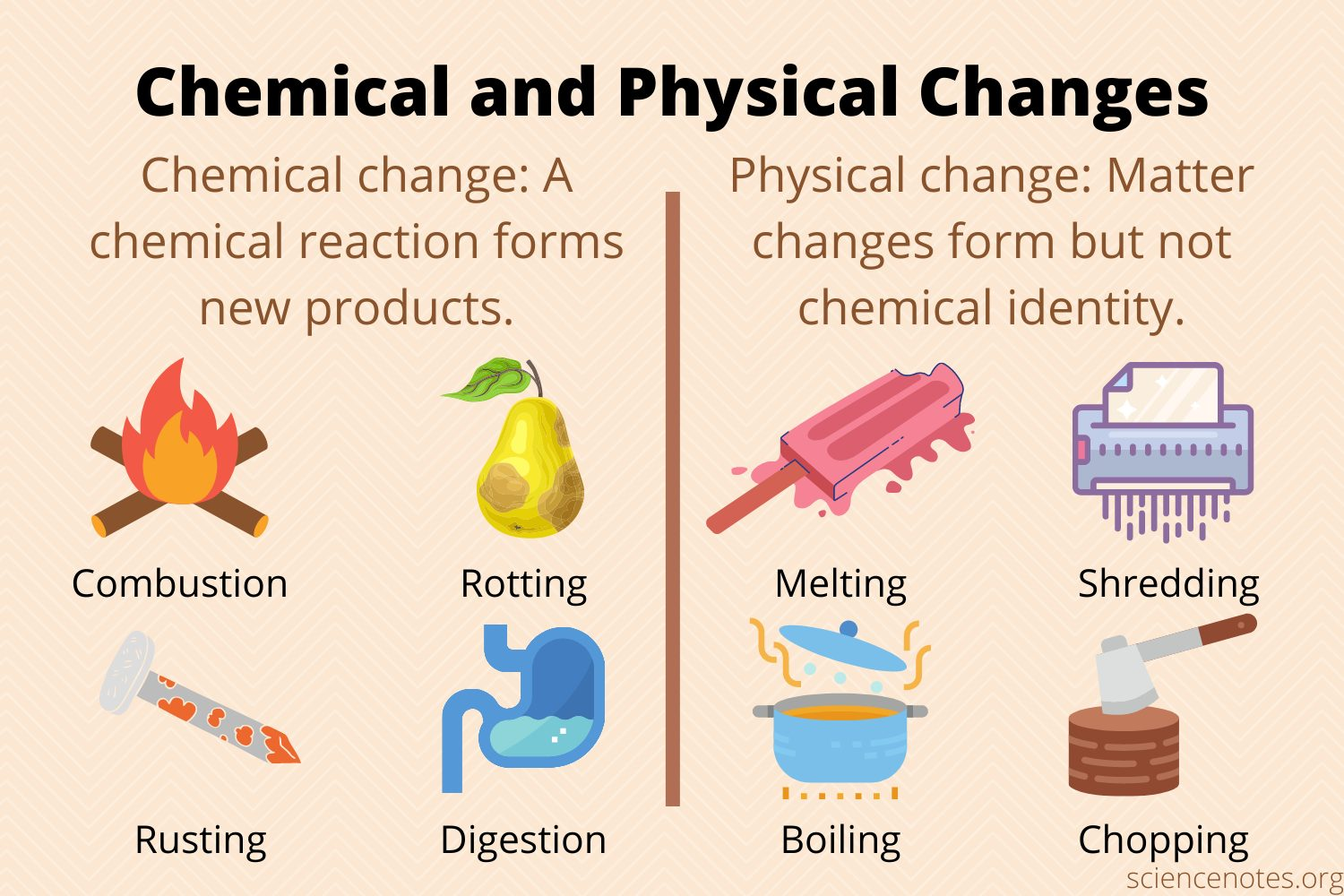
The changes that matter experiences are classified as either physical or chemical
Physical Chance - any alteration to the size, shape, or state of a substance; the final changes take place without altering the substance's molecular composition; the final substance is made of the same matter as before the change
Example: when an ice cube melts, it has undergone a change from solid to liquid, but it's still the same substance - water
Chemical Change - occurs when matter changes into a new substance and has a new chemical property; they do alter the molecular makeup of the substance; the final substance is not made of the same matter as before the change
Example: burning a log changes it from a solid piece of wood to ash and gases; you can't change the ash back into the solid log and therefore a chemical change has occurred
Signs when something has undergone a chemical change...
Change in color - this is similar to what occurs when you leave a sliced apple out of the refrigerator and it turns brown
Change in odor - a smell is given off and it can be an unpleasant smell, like rotten food
Formation of a gas - mixing two substances that emit a gas such as vinegar and baking soda, which releases bubbles that show that gas has formed
Formation of a solid - mixing two substances that form a new solid such as when ice melting pellets combine with baking soda in a solution to create chalk which is a precipitate (a new solid that is formed during a chemical reaction)
Change in energy - a chemical reaction that can be in the form of heat and/or light that releases energy
A physical or chemical reaction that releases heat and energy is exothermic, like making ice cubes.
A physical or chemical reaction that absorbs heat and/or energy to complete its reaction is endothermic, like boiling water or melting ice cubes.
Properties of Matter!
Properties of Matter!
What is matter?
Matter is anything that occupies space and has mass. If you can see, touch, taste, smell, or feel it then it's matter.
A property describes how an object looks, feels, or acts. All properties of matter are classified as either physical or chemical.
Physical Property - is usually observed with our senses
Examples...
Color - the quality of an object or substance with respect to the reflection of light
Size - an object's overall dimensions
Volume - the amount of space a substance or object occupies
Density - the ratio of mass and volume in a substance
Boiling Point/Melting Point - the temperature at which something boils or melts
Magnetism - whether or not something is magnetic
Solubility - how easily something dissolves in another substance
Physical properties are broken down into two different categories...
Intensive - they do not depend on the amount of the substance present; ex. the density of a substance at room temperature is the same no matter how much of the substance that you have
Other examples of intensive properties: color, odor, temperature, freezing point, melting point, boiling point, density, state of matter, malleability, and ductility
Extensive - they depend on the amount of matter being measured; ex. mass, length, and volume measures depending on how much of the object that you have
Other examples of extensive properties: size, length, width, volume, mass, and weight
Extensive properties can be added together. For example, two pennies will have more mass than one penny because the mass of two is greater than the mass of one.

Chemical Property - any characteristic that can be determined only by changing a substance's identity, possibly through a chemical reaction
Examples...
Reactivity - the likelihood of a substance to undergo a chemical reaction
Toxicity - how poisonous or damaging a chemical substance may be to organisms
Flammability - whether a substance will burn when exposed to a flame
Combustibility - the measure of how easily a substance will burn in oxygen
When determining the properties of matter, if you can identify the properties of the substance without changing it, then it is a physical property. If not, it is a chemical property.
The changes that matter experiences are classified as either physical or chemical

Physical Chance - any alteration to the size, shape, or state of a substance; the final changes take place without altering the substance's molecular composition; the final substance is made of the same matter as before the change
Example: when an ice cube melts, it has undergone a change from solid to liquid, but it's still the same substance - water
Chemical Change - occurs when matter changes into a new substance and has a new chemical property; they do alter the molecular makeup of the substance; the final substance is not made of the same matter as before the change
Example: burning a log changes it from a solid piece of wood to ash and gases; you can't change the ash back into the solid log and therefore a chemical change has occurred
Signs when something has undergone a chemical change...
Change in color - this is similar to what occurs when you leave a sliced apple out of the refrigerator and it turns brown
Change in odor - a smell is given off and it can be an unpleasant smell, like rotten food
Formation of a gas - mixing two substances that emit a gas such as vinegar and baking soda, which releases bubbles that show that gas has formed
Formation of a solid - mixing two substances that form a new solid such as when ice melting pellets combine with baking soda in a solution to create chalk which is a precipitate (a new solid that is formed during a chemical reaction)
Change in energy - a chemical reaction that can be in the form of heat and/or light that releases energy
A physical or chemical reaction that releases heat and energy is exothermic, like making ice cubes.
A physical or chemical reaction that absorbs heat and/or energy to complete its reaction is endothermic, like boiling water or melting ice cubes.

 Knowt
Knowt
