Electromagnetic Waves
ELECTROMAGNETIC WAVES
wave created as a result of vibrations between an electric and magnetic field
can travel without a medium
has the same speed (speed of light)
has no matter
all EM waves are radiation
higher frequency = shorter wavelength
lower frequency = longer wavelength
electric & magnetic fields oscillate perpendicular to each other and to the direction of the propagating wave
travel in vacuum at a speed of 3.0 x 10⁸ m/s (denoted as c = speed of light)
speed, frequency and wavelength are related by the ff equation:
v = λf
v = speed : m/s
λ = wavelength : m
f = frequency : Hertz (Hz)
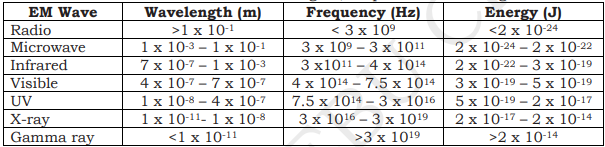
ELECTROMAGNETIC SPECTRUM
continuum of EM waves arranged according to frequency and wavelength
according to increasing frequency: radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, gamma rays.
different types are defined by the amount of energy carried or possessed by the photons
waves with short wavelengths have high energy and can be very dangerous
there is no sharp dividing line between one kind of wave and the next
Photons
bundles of wave energy
energy of a photon is given by the equation:
E = hf
E = energy of a photon (Photon Energy)
h = Planck’s constant
= 6.63 x 10⁻³⁴ J/s
J = joule
f = frequency
COMMON PROPERTIES OF EM WAVES
carry energy from one place to another
do not refuse a medium to travel
they show reflection, refraction, absorption, and interference
transverse waves
GAMMA RAYS |
|---|
Wavelength: less than 0.01 nm
Frequency (Hz): more than 10 EHz
Photon Energy (eV): 100 keV - 300+ GeV
X-RAYS |
|---|
Wavelength: 0.01 - 10 nm
Frequency (Hz): 30 EHz - 30 PHz
Photon Energy (eV): 120 eV - 120 keV
ULTRAVIOLET |
|---|
Wavelength: 10 nm - 400 nm
Frequency (Hz): 30 PHz - 790 THz
Photon Energy (eV): 3eV - 124 eV
VISIBLE LIGHT |
|---|
Wavelength: 390 nm - 750 nm
Frequency (Hz): 790 THz - 405 THz
Photon Energy (eV): 1.7 eV - 3.3 eV
INFRARED |
|---|
Wavelength: 750 nm - 1 mm
Frequency (Hz): 405 THz - 300 GHz
Photon Energy (eV): 1.24 meV - 1.7 eV
MICROWAVES |
|---|
Wavelength: 1 mm - 1 meter
Frequency (Hz): 300 GHz - 300 MHz
Photon Energy (eV): 1.24 μeV - 1.24 meV
* μ = micro
RADIO WAVES |
|---|
Wavelength: 1 mm - km
Frequency (Hz): 300 GHz - 3 Hz
Photon Energy (eV): 12.4 feV - 1.24 meV
TYPES OF ELECTROMAGNETIC RADIATION
Radio
used to broadcast radio and television
Microwaves
used in cooking, radar, telephone, and other signals
Infrared
transmits heat from sun, fires, radiators
Visible Light
makes things able to be seen
Ultraviolet
absorbed by the skin, used in fluorescent tubes
X-rays
used to view inside of bodies and objects
Gamma rays
used in medicine for killing cancer cells
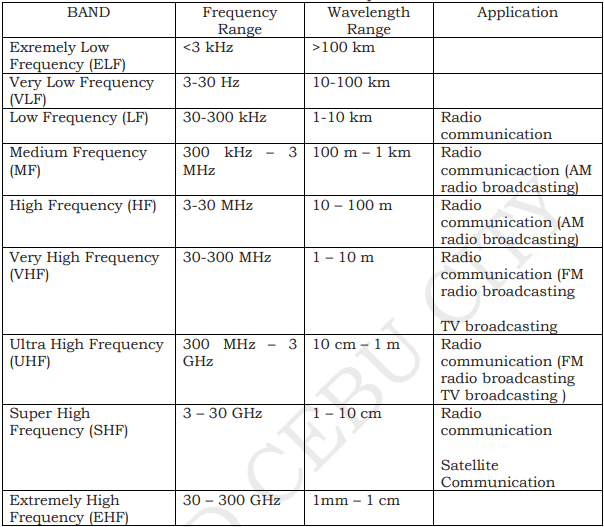
RADIO WAVES
have the longest wavelengths in the EM spectrum
range from the length of a football to larger than our planet
Heinrich Hertz proved the existence of radio waves in the late 1880s
used to transmit sound and picture information over long distances
Low frequency waves are suitable for communication over great distances. But the curvature of the earth limits the range to about 80 kilometers. To extend the range, a repeater is used.
High frequency waves can be reflected by the ionosphere. This enables the waves to be transmitted over great distances.
Medium and high frequency waves are used for broadcasting by local radio stations.
CHARACTERISTICS
not line of sight
can pass through walls
longer range
not light sensitive
DISADVANTAGES
communication devices that make use of the same frequencies interfere with their transmission
easier to “eavesdrop” since signals are transmitted in a space rather than a wire
more costly than infrared
MICROWAVES
have smaller wavelengths than radio waves
used in satellite communications, radar, television transmission, and cooking.
wavelengths ranging from as long as one meter to as short as one millimeter
the prefix “micro-” in “microwave” is not meant to suggest a wavelength in the micrometer range. It indicates that microwaves are “small” because have shorter wavelengths as compared to waves used in typical radio broadcasting
APPLICATIONS
Terrestrial Communication
Satellite Communication
Microwave Oven
a part of the oven produces microwaves
the microwaves are sent to the reflecting fan
the microwaves are reflected in many directions by the fan and the walls of the microwave oven
as microwaves pass through the food, they transfer energy to the water molecules in the form of heat. this will cook the food
INFRARED
lies beyond the red end of the visible light
emitted by all objects.
the amount and wavelength of radiation depend on temperature
below 500C, an object emits only infrared radiation
above 500C, an object glows and emits both infrared and some visible light
typical television remote control uses infrared energy at a wavelength around 940 nanometers
infrared lamps heat lamps often emit both visible and infrared energy at wavelengths between 500 nm to 3000 nm in length. They can be used to heat the bathroom or keep food warm, they can also keep small animals and reptiles warm or even to keep eggs warm so they can hatch
divided into near-, mid-, and far-infrared. The region from 8 to 15 microns (µm) is referred to by Earth scientists as thermal infrared since these wavelengths are best for studying the longwave thermal energy radiating from our planet
DISCOVERY OF INFRARED
In 1800, William Herschel conducted an experiment measuring the difference in temperature between the colors in the visible spectrum. He placed thermometers within each color of the visible spectrum. The results showed an increase in temperature from blue to red. When he noticed an even warmer temperature measurement just beyond the red end of the visible spectrum, Herschel had discovered infrared light.
APPLICATIONS
Thermal Imaging
Infrared Photographs
Infrared Scanners
VISIBLE LIGHT
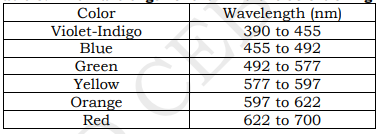
The visible light spectrum is the segment of the electromagnetic spectrum that the human eye can view. More simply, this range of wavelengths is called visible light. Typically, the human eye can detect wavelengths from 380 to 700 nanometers.
As the full spectrum of visible light travels through a prism, the wavelengths separate into the colors of the rainbow because each color is a different wavelength. Violet has the shortest wavelength, at around 380 nanometers, and red has the longest wavelength, at around 700 nanometers.
lies in between the infrared and ultraviolet rays
thinnest slice in the spectrum
only EM wave perceived by the human eye
white light, like that of the sunlight, is made up of a variety of colors arranged as follows: red, orange, yellow, green, blue, indigo, violet.
Isaac Newton's experiment in 1665 showed that a prism bends visible light and that each color refracts at a slightly different angle depending on the wavelength of the color.
do not distinguishably separate between colors but continuously changing from red-violet
EM wave can be bent when traveling from one medium to another
violet bends most
Close examination of the visible-light spectrum from our Sun and other stars reveals a pattern of dark lines—called absorption lines. These patterns can provide important scientific clues that reveal hidden properties of objects throughout the universe. Certain elements in the Sun's atmosphere absorb certain colors of light. These patterns of lines within spectra act like fingerprints for atoms and molecules. Looking at the Sun's spectrum, for example, the fingerprints for elements are clear to those knowledgeable about those patterns.
Patterns are also evident in a graph of an object's reflectance. Elements, molecules, and even cell structures have unique signatures of reflectance. A graph of an object's reflectance across a spectrum is called a spectral signature.
used as proof for CMB (Cosmic Microwave Background)
sky is blue because of chemical components in the atmosphere (nitrogen) that refracts blue light the most
sunlight emits many EM waves like UV, infrared, etc but the only visible to us is visible light
Our eyes are sensitive to electromagnetic waves of wavelengths that ranges from 4 x 10⁻⁷ m to 7 x 10⁻⁷ m.
ULTRAVIOLET
has shorter wavelengths than visible light. Although UV waves are invisible to the human eye, some insects, such as bumblebees, can see them. This is similar to how a dog can hear the sound of a whistle just outside the hearing range of humans.
wavelength shorter than that of visible light in the range 10 nm to 400 nm
Solar UV radiation is commonly subdivided into three regions: UV-A (320–400 nm), UV-B (290–320 nm), and UV-C (220–290 nm), ranked from long to shorter wavelengths (from smaller to larger energies). Most UV-B and all UV-C is absorbed by ozone (O3) molecules in the upper atmosphere. Consequently, 99% of the solar UV radiation reaching the Earth’s surface is UV-A.
There are other schemes for dividing UV into different categories, another common one is: near-ultraviolet (NUV – 300-400 nm), middle ultraviolet (MUV – 200- 300 nm), far ultraviolet (FUV – 200-122 nm), and extreme ultraviolet (EUV- 121-10 nm).
TYPES OF UV RAYS
UV-A |
|---|
tanning, wrinkles |
UV-B |
|---|
harmful rays that cause sunburn and cancerexposure to UV-B rays increases the risk of DNA and other cellular damage in living organismsabout 95% UV-B rays are absorbed by ozone in the earth’s atmosphere |
UV-C |
|---|
most harmfulalmost completely absorbed by our atmospheresterilization |
SPF 15 and above for sun protection
ultraviolet rays can damage tissue, burn the skin and damage the eyes
Scientists studying astronomical objects commonly refer to different subdivisions of ultraviolet radiation:
near ultraviolet (NUV)
middle ultraviolet (MUV)
far ultraviolet (FUV)
extreme ultraviolet (EUV)
USES
production of vitamin D in our skin
sterilization of water in drinking fountains
identifying original from fake banknotes
used to sterilize medical equipment
dental tools
sunbed
DISCOVERY OF ULTRAVIOLET
In 1801, Johann Ritter conducted an experiment to investigate the existence of energy beyond the violet end of the visible spectrum. Knowing that photographic paper would turn black more rapidly in blue light than in red light, he exposed the paper to light beyond violet. Sure enough, the paper turned black, proving the existence of ultraviolet light.
X-RAYS
have much higher energy and much shorter wavelengths than ultraviolet light, and scientists usually refer to x-rays in terms of their energy rather than their wavelength. This is partially because x-rays have very small wavelengths, between 0.03 and 3 nanometers, so small that some x-rays are no bigger than a single atom of many elements.
Our Sun's radiation peaks in the visual range, but the Sun's corona is much hotter and radiates mostly x-rays. To study the corona, scientists use data collected by x-ray detectors on satellites in orbit around the Earth. Japan's Hinode spacecraft produced these x-ray images of the Sun that allow scientists to see and record the energy flows within the corona.
X-rays are electromagnetic waves with wavelengths in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 3×1016 Hz to 3×1019 Hz. X-rays come just after the ultraviolet rays. They are of shorter wavelengths but carry higher energy than the ultraviolet. They are produced using an X-ray tube. They are emitted when fast moving electrons hit a metal target.
Long wavelength X-rays can penetrate the flesh but not the bones. They are used in X-ray photographs to help doctors look inside the body. They are useful in diagnosing bone fractures and tumors.
Short wavelength X-rays can penetrate even through metals. They are used in industry to inspect welded joints for faults.
All X-rays are dangerous because they can damage healthy living cells of the body. This is the reason why frequent exposure to X-rays should be avoided. Too much exposure to X-rays can damage body tissues and can cause cancer.
DISCOVERY OF X-RAYS
X-rays were first observed and documented in 1895 by German scientist Wilhelm Conrad Roentgen. He discovered that firing streams of x-rays through arms and hands created detailed images of the bones inside. When you get an x-ray taken, x-ray sensitive film is put on one side of your body, and x-rays are shot through you. Because bones are dense and absorb more x-rays than skin does, shadows of the bones are left on the x-ray film while the skin appears transparent.
first clinical x-ray taken by Wilhelm Roentgen on December 22, 1895, of his wife’s hand, showing wedding ring and bones of fingers
15 minute exposure (his wife)
USES
medical imaging
security
radiation therapy
checking authenticity of art pieces
engineering applications
industries
research and development
astronomy
GAMMA RAYS
lie at the other end of the electromagnetic spectrum
shortest in wavelength and highest in frequency
carry the highest amount of energy, thus, they are more dangerous.
emitted by stars and some radioactive substances.
can only be blocked with lead and thick concrete.
ionizing radiation and are thus biologically hazardous.
classically produced by the decay from high energy states of atomic nuclei, a process called gamma decay, but are also created by other processes.
PAUL VILLARD |
|---|
a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium during its gamma decay. Villard’s radiation was named “gamma rays” by Ernest Rutherford in 1903. |
have the smallest wavelengths and the most energy of any wave in the electromagnetic spectrum
produced by the hottest and most energetic objects in the universe, such as neutron stars and pulsars, supernova explosions, and regions around black holes.
On Earth, gamma waves are generated by nuclear explosions, lightning, and the less dramatic activity of radioactive decay.
COBALT-60 (CO-60) |
|---|
used medically for radiotherapy. it is used to treat cancer. |
USES
used to treat cancer (radiotherapy)
used in sterilizing medical equipment
nuclear industry
Electromagnetic Waves
ELECTROMAGNETIC WAVES
wave created as a result of vibrations between an electric and magnetic field
can travel without a medium
has the same speed (speed of light)
has no matter
all EM waves are radiation
higher frequency = shorter wavelength
lower frequency = longer wavelength
electric & magnetic fields oscillate perpendicular to each other and to the direction of the propagating wave
travel in vacuum at a speed of 3.0 x 10⁸ m/s (denoted as c = speed of light)
speed, frequency and wavelength are related by the ff equation:
v = λf
v = speed : m/s
λ = wavelength : m
f = frequency : Hertz (Hz)
ELECTROMAGNETIC SPECTRUM
continuum of EM waves arranged according to frequency and wavelength
according to increasing frequency: radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, gamma rays.
different types are defined by the amount of energy carried or possessed by the photons
waves with short wavelengths have high energy and can be very dangerous
there is no sharp dividing line between one kind of wave and the next
Photons
bundles of wave energy
energy of a photon is given by the equation:
E = hf
E = energy of a photon (Photon Energy)
h = Planck’s constant
= 6.63 x 10⁻³⁴ J/s
J = joule
f = frequency
COMMON PROPERTIES OF EM WAVES
carry energy from one place to another
do not refuse a medium to travel
they show reflection, refraction, absorption, and interference
transverse waves
GAMMA RAYS |
|---|
Wavelength: less than 0.01 nm
Frequency (Hz): more than 10 EHz
Photon Energy (eV): 100 keV - 300+ GeV
X-RAYS |
|---|
Wavelength: 0.01 - 10 nm
Frequency (Hz): 30 EHz - 30 PHz
Photon Energy (eV): 120 eV - 120 keV
ULTRAVIOLET |
|---|
Wavelength: 10 nm - 400 nm
Frequency (Hz): 30 PHz - 790 THz
Photon Energy (eV): 3eV - 124 eV
VISIBLE LIGHT |
|---|
Wavelength: 390 nm - 750 nm
Frequency (Hz): 790 THz - 405 THz
Photon Energy (eV): 1.7 eV - 3.3 eV
INFRARED |
|---|
Wavelength: 750 nm - 1 mm
Frequency (Hz): 405 THz - 300 GHz
Photon Energy (eV): 1.24 meV - 1.7 eV
MICROWAVES |
|---|
Wavelength: 1 mm - 1 meter
Frequency (Hz): 300 GHz - 300 MHz
Photon Energy (eV): 1.24 μeV - 1.24 meV
* μ = micro
RADIO WAVES |
|---|
Wavelength: 1 mm - km
Frequency (Hz): 300 GHz - 3 Hz
Photon Energy (eV): 12.4 feV - 1.24 meV
TYPES OF ELECTROMAGNETIC RADIATION
Radio
used to broadcast radio and television
Microwaves
used in cooking, radar, telephone, and other signals
Infrared
transmits heat from sun, fires, radiators
Visible Light
makes things able to be seen
Ultraviolet
absorbed by the skin, used in fluorescent tubes
X-rays
used to view inside of bodies and objects
Gamma rays
used in medicine for killing cancer cells
RADIO WAVES
have the longest wavelengths in the EM spectrum
range from the length of a football to larger than our planet
Heinrich Hertz proved the existence of radio waves in the late 1880s
used to transmit sound and picture information over long distances
Low frequency waves are suitable for communication over great distances. But the curvature of the earth limits the range to about 80 kilometers. To extend the range, a repeater is used.
High frequency waves can be reflected by the ionosphere. This enables the waves to be transmitted over great distances.
Medium and high frequency waves are used for broadcasting by local radio stations.
CHARACTERISTICS
not line of sight
can pass through walls
longer range
not light sensitive
DISADVANTAGES
communication devices that make use of the same frequencies interfere with their transmission
easier to “eavesdrop” since signals are transmitted in a space rather than a wire
more costly than infrared
MICROWAVES
have smaller wavelengths than radio waves
used in satellite communications, radar, television transmission, and cooking.
wavelengths ranging from as long as one meter to as short as one millimeter
the prefix “micro-” in “microwave” is not meant to suggest a wavelength in the micrometer range. It indicates that microwaves are “small” because have shorter wavelengths as compared to waves used in typical radio broadcasting
APPLICATIONS
Terrestrial Communication
Satellite Communication
Microwave Oven
a part of the oven produces microwaves
the microwaves are sent to the reflecting fan
the microwaves are reflected in many directions by the fan and the walls of the microwave oven
as microwaves pass through the food, they transfer energy to the water molecules in the form of heat. this will cook the food
INFRARED
lies beyond the red end of the visible light
emitted by all objects.
the amount and wavelength of radiation depend on temperature
below 500C, an object emits only infrared radiation
above 500C, an object glows and emits both infrared and some visible light
typical television remote control uses infrared energy at a wavelength around 940 nanometers
infrared lamps heat lamps often emit both visible and infrared energy at wavelengths between 500 nm to 3000 nm in length. They can be used to heat the bathroom or keep food warm, they can also keep small animals and reptiles warm or even to keep eggs warm so they can hatch
divided into near-, mid-, and far-infrared. The region from 8 to 15 microns (µm) is referred to by Earth scientists as thermal infrared since these wavelengths are best for studying the longwave thermal energy radiating from our planet
DISCOVERY OF INFRARED
In 1800, William Herschel conducted an experiment measuring the difference in temperature between the colors in the visible spectrum. He placed thermometers within each color of the visible spectrum. The results showed an increase in temperature from blue to red. When he noticed an even warmer temperature measurement just beyond the red end of the visible spectrum, Herschel had discovered infrared light.
APPLICATIONS
Thermal Imaging
Infrared Photographs
Infrared Scanners
VISIBLE LIGHT
The visible light spectrum is the segment of the electromagnetic spectrum that the human eye can view. More simply, this range of wavelengths is called visible light. Typically, the human eye can detect wavelengths from 380 to 700 nanometers.
As the full spectrum of visible light travels through a prism, the wavelengths separate into the colors of the rainbow because each color is a different wavelength. Violet has the shortest wavelength, at around 380 nanometers, and red has the longest wavelength, at around 700 nanometers.
lies in between the infrared and ultraviolet rays
thinnest slice in the spectrum
only EM wave perceived by the human eye
white light, like that of the sunlight, is made up of a variety of colors arranged as follows: red, orange, yellow, green, blue, indigo, violet.
Isaac Newton's experiment in 1665 showed that a prism bends visible light and that each color refracts at a slightly different angle depending on the wavelength of the color.
do not distinguishably separate between colors but continuously changing from red-violet
EM wave can be bent when traveling from one medium to another
violet bends most
Close examination of the visible-light spectrum from our Sun and other stars reveals a pattern of dark lines—called absorption lines. These patterns can provide important scientific clues that reveal hidden properties of objects throughout the universe. Certain elements in the Sun's atmosphere absorb certain colors of light. These patterns of lines within spectra act like fingerprints for atoms and molecules. Looking at the Sun's spectrum, for example, the fingerprints for elements are clear to those knowledgeable about those patterns.
Patterns are also evident in a graph of an object's reflectance. Elements, molecules, and even cell structures have unique signatures of reflectance. A graph of an object's reflectance across a spectrum is called a spectral signature.
used as proof for CMB (Cosmic Microwave Background)
sky is blue because of chemical components in the atmosphere (nitrogen) that refracts blue light the most
sunlight emits many EM waves like UV, infrared, etc but the only visible to us is visible light
Our eyes are sensitive to electromagnetic waves of wavelengths that ranges from 4 x 10⁻⁷ m to 7 x 10⁻⁷ m.
ULTRAVIOLET
has shorter wavelengths than visible light. Although UV waves are invisible to the human eye, some insects, such as bumblebees, can see them. This is similar to how a dog can hear the sound of a whistle just outside the hearing range of humans.
wavelength shorter than that of visible light in the range 10 nm to 400 nm
Solar UV radiation is commonly subdivided into three regions: UV-A (320–400 nm), UV-B (290–320 nm), and UV-C (220–290 nm), ranked from long to shorter wavelengths (from smaller to larger energies). Most UV-B and all UV-C is absorbed by ozone (O3) molecules in the upper atmosphere. Consequently, 99% of the solar UV radiation reaching the Earth’s surface is UV-A.
There are other schemes for dividing UV into different categories, another common one is: near-ultraviolet (NUV – 300-400 nm), middle ultraviolet (MUV – 200- 300 nm), far ultraviolet (FUV – 200-122 nm), and extreme ultraviolet (EUV- 121-10 nm).
TYPES OF UV RAYS
UV-A |
|---|
tanning, wrinkles |
UV-B |
|---|
harmful rays that cause sunburn and cancerexposure to UV-B rays increases the risk of DNA and other cellular damage in living organismsabout 95% UV-B rays are absorbed by ozone in the earth’s atmosphere |
UV-C |
|---|
most harmfulalmost completely absorbed by our atmospheresterilization |
SPF 15 and above for sun protection
ultraviolet rays can damage tissue, burn the skin and damage the eyes
Scientists studying astronomical objects commonly refer to different subdivisions of ultraviolet radiation:
near ultraviolet (NUV)
middle ultraviolet (MUV)
far ultraviolet (FUV)
extreme ultraviolet (EUV)
USES
production of vitamin D in our skin
sterilization of water in drinking fountains
identifying original from fake banknotes
used to sterilize medical equipment
dental tools
sunbed
DISCOVERY OF ULTRAVIOLET
In 1801, Johann Ritter conducted an experiment to investigate the existence of energy beyond the violet end of the visible spectrum. Knowing that photographic paper would turn black more rapidly in blue light than in red light, he exposed the paper to light beyond violet. Sure enough, the paper turned black, proving the existence of ultraviolet light.
X-RAYS
have much higher energy and much shorter wavelengths than ultraviolet light, and scientists usually refer to x-rays in terms of their energy rather than their wavelength. This is partially because x-rays have very small wavelengths, between 0.03 and 3 nanometers, so small that some x-rays are no bigger than a single atom of many elements.
Our Sun's radiation peaks in the visual range, but the Sun's corona is much hotter and radiates mostly x-rays. To study the corona, scientists use data collected by x-ray detectors on satellites in orbit around the Earth. Japan's Hinode spacecraft produced these x-ray images of the Sun that allow scientists to see and record the energy flows within the corona.
X-rays are electromagnetic waves with wavelengths in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 3×1016 Hz to 3×1019 Hz. X-rays come just after the ultraviolet rays. They are of shorter wavelengths but carry higher energy than the ultraviolet. They are produced using an X-ray tube. They are emitted when fast moving electrons hit a metal target.
Long wavelength X-rays can penetrate the flesh but not the bones. They are used in X-ray photographs to help doctors look inside the body. They are useful in diagnosing bone fractures and tumors.
Short wavelength X-rays can penetrate even through metals. They are used in industry to inspect welded joints for faults.
All X-rays are dangerous because they can damage healthy living cells of the body. This is the reason why frequent exposure to X-rays should be avoided. Too much exposure to X-rays can damage body tissues and can cause cancer.
DISCOVERY OF X-RAYS
X-rays were first observed and documented in 1895 by German scientist Wilhelm Conrad Roentgen. He discovered that firing streams of x-rays through arms and hands created detailed images of the bones inside. When you get an x-ray taken, x-ray sensitive film is put on one side of your body, and x-rays are shot through you. Because bones are dense and absorb more x-rays than skin does, shadows of the bones are left on the x-ray film while the skin appears transparent.
first clinical x-ray taken by Wilhelm Roentgen on December 22, 1895, of his wife’s hand, showing wedding ring and bones of fingers
15 minute exposure (his wife)
USES
medical imaging
security
radiation therapy
checking authenticity of art pieces
engineering applications
industries
research and development
astronomy
GAMMA RAYS
lie at the other end of the electromagnetic spectrum
shortest in wavelength and highest in frequency
carry the highest amount of energy, thus, they are more dangerous.
emitted by stars and some radioactive substances.
can only be blocked with lead and thick concrete.
ionizing radiation and are thus biologically hazardous.
classically produced by the decay from high energy states of atomic nuclei, a process called gamma decay, but are also created by other processes.
PAUL VILLARD |
|---|
a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium during its gamma decay. Villard’s radiation was named “gamma rays” by Ernest Rutherford in 1903. |
have the smallest wavelengths and the most energy of any wave in the electromagnetic spectrum
produced by the hottest and most energetic objects in the universe, such as neutron stars and pulsars, supernova explosions, and regions around black holes.
On Earth, gamma waves are generated by nuclear explosions, lightning, and the less dramatic activity of radioactive decay.
COBALT-60 (CO-60) |
|---|
used medically for radiotherapy. it is used to treat cancer. |
USES
used to treat cancer (radiotherapy)
used in sterilizing medical equipment
nuclear industry
 Knowt
Knowt