
3 | Electron Configuration
An electron configuration shows the occupation of orbitals by electrons for a particular atom
the electron configuration for a ground-state hydrogen atom is this:
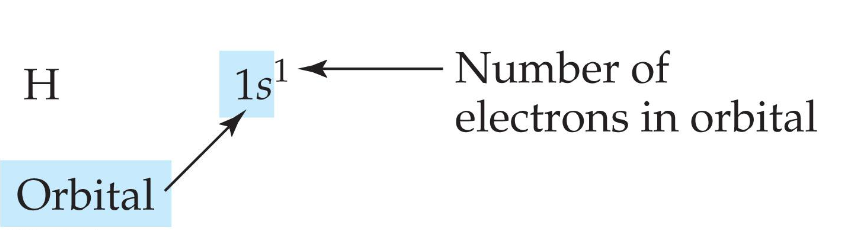
Another way to represent this information is with an orbital diagram, which gives similar information but shows the electrons as arrows in a box representing the orbital.
The orbital diagram for a ground-state hydrogen atom is as follows:
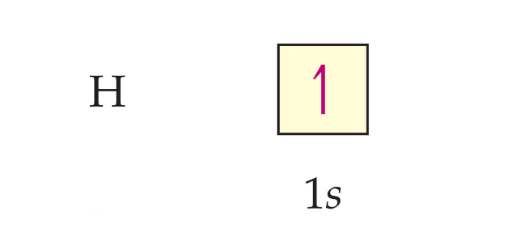
In orbital diagrams, the direction of the arrow (pointing up or pointing down) represents electron spin, a fundamental property of electrons.
The Pauli exclusion principle states that orbitals may hold no more than two electrons with opposing spins. We symbolize this as two arrows pointing in opposite directions.

In multi-electron atoms, the subshells within a principal shell do not have the same energy because of electron–electron interactions. Different subshells within the same principal shell have different energies. The 4s subshell is lower in energy than the 3d subshell, even though its principal quantum number is higher.
Another example includes carbon which has 6 electrons
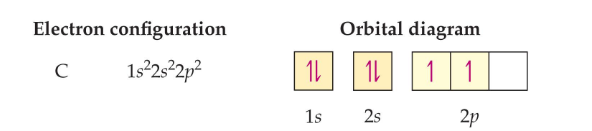
This is the result of Hund’s rule: When filling orbitals of equal energy, electrons fill them singly first, with parallel spins.
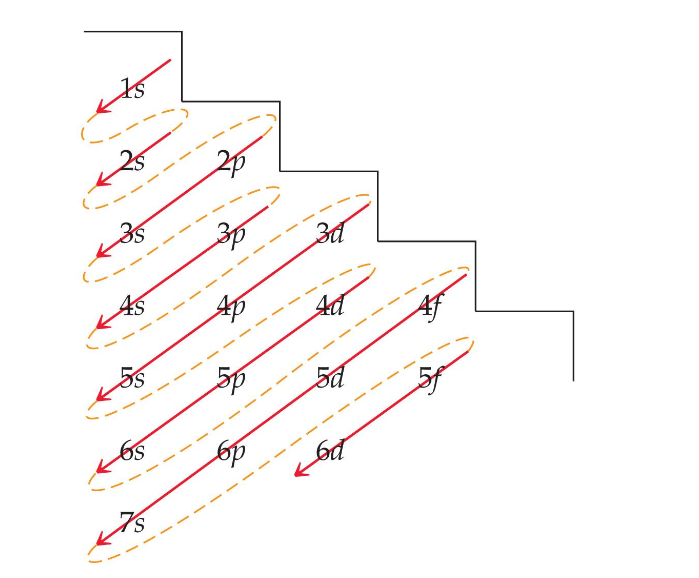
Lower-energy orbitals fill before higher-energy orbitals.
Orbitals can hold no more than two electrons each. When two electrons occupy the same orbital, they must have opposing spins. This is known as the Pauli exclusion principle.
When orbitals of identical energy are available, all of these are first occupied singly by electrons with parallel spins rather than electrons in pairs. This is known as Hund’s rule.
noble gas notation |
in order to be able to do noble gas notation you first have to do fill
the noble gas has to have 1 less than the protons
He = 2
Ne = 10
Ar = 18
Kr = 36
3 | Electron Configuration
An electron configuration shows the occupation of orbitals by electrons for a particular atom
the electron configuration for a ground-state hydrogen atom is this:

Another way to represent this information is with an orbital diagram, which gives similar information but shows the electrons as arrows in a box representing the orbital.
The orbital diagram for a ground-state hydrogen atom is as follows:

In orbital diagrams, the direction of the arrow (pointing up or pointing down) represents electron spin, a fundamental property of electrons.
The Pauli exclusion principle states that orbitals may hold no more than two electrons with opposing spins. We symbolize this as two arrows pointing in opposite directions.

In multi-electron atoms, the subshells within a principal shell do not have the same energy because of electron–electron interactions. Different subshells within the same principal shell have different energies. The 4s subshell is lower in energy than the 3d subshell, even though its principal quantum number is higher.
Another example includes carbon which has 6 electrons

This is the result of Hund’s rule: When filling orbitals of equal energy, electrons fill them singly first, with parallel spins.

Lower-energy orbitals fill before higher-energy orbitals.
Orbitals can hold no more than two electrons each. When two electrons occupy the same orbital, they must have opposing spins. This is known as the Pauli exclusion principle.
When orbitals of identical energy are available, all of these are first occupied singly by electrons with parallel spins rather than electrons in pairs. This is known as Hund’s rule.
noble gas notation |
in order to be able to do noble gas notation you first have to do fill
the noble gas has to have 1 less than the protons
He = 2
Ne = 10
Ar = 18
Kr = 36
 Knowt
Knowt