
Half-life
Radioactivity is a totally random process
Radioactive substances give out radiation from the nuclei of their atoms-no matter what
This radiation can be measured with a Geiger-Muller tube and counter, which records the count-rate-the number of radiation counts reaching it per second
Radioactive decay is entirely random. So you can’t predict exactly which nucleus in a sample will decay next, or when any one of them will decay
But you can find out the time it takes for the amount of radiation emitted by a source to halve, this is known as the half-life. It can be used to make predictions about radioactive sources, even though their decays are random
Half-life can be used to find the rate at which a source decays-its ACTIVITY. Activity is measured in becquerels, Bq
The radioactivity of a source decreases over time
Each time a radioactive nucleus decays to become a stable nucleus, the activity as a whole will decrease
For some isotopes it takes just a few hours before nearly all the unstable nuclei have decayed, whilst others last for millions of years
The problem with trying to measure this is that the activity never reaches zero, which is why we have to use the idea of half-life to measure how quickly the activity drops off
The half-life is the time taken for the number of radioactive nuclei in an isotope to halve
It is also the time taken for the activity, and so count-rate, to halve. A short half-life means the activity falls quickly, because the nuclei are very unstable and rapidly decay. Sources with a short half-life are dangerous because of the high amount of radiation they emit at the start, but they quickly become safe
A long half-life means the activity falls more slowly because most of the nuclei don’t decay for a long time-the source just sits there, releasing small amounts of radiation for a long time. This can be dangerous because nearby areas are exposed to radiation for millions of years
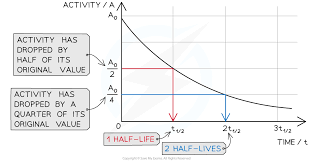
You can measure half-life using a graph
If you plot a graph of activity against time, it will always be shaped like the one to the right
The half-life is found from the graph by finding the time interval on the bottom axis corresponding to a halving of the activity on the vertical axis
Half-life
Radioactivity is a totally random process
Radioactive substances give out radiation from the nuclei of their atoms-no matter what
This radiation can be measured with a Geiger-Muller tube and counter, which records the count-rate-the number of radiation counts reaching it per second
Radioactive decay is entirely random. So you can’t predict exactly which nucleus in a sample will decay next, or when any one of them will decay
But you can find out the time it takes for the amount of radiation emitted by a source to halve, this is known as the half-life. It can be used to make predictions about radioactive sources, even though their decays are random
Half-life can be used to find the rate at which a source decays-its ACTIVITY. Activity is measured in becquerels, Bq
The radioactivity of a source decreases over time
Each time a radioactive nucleus decays to become a stable nucleus, the activity as a whole will decrease
For some isotopes it takes just a few hours before nearly all the unstable nuclei have decayed, whilst others last for millions of years
The problem with trying to measure this is that the activity never reaches zero, which is why we have to use the idea of half-life to measure how quickly the activity drops off
The half-life is the time taken for the number of radioactive nuclei in an isotope to halve
It is also the time taken for the activity, and so count-rate, to halve. A short half-life means the activity falls quickly, because the nuclei are very unstable and rapidly decay. Sources with a short half-life are dangerous because of the high amount of radiation they emit at the start, but they quickly become safe
A long half-life means the activity falls more slowly because most of the nuclei don’t decay for a long time-the source just sits there, releasing small amounts of radiation for a long time. This can be dangerous because nearby areas are exposed to radiation for millions of years
You can measure half-life using a graph
If you plot a graph of activity against time, it will always be shaped like the one to the right
The half-life is found from the graph by finding the time interval on the bottom axis corresponding to a halving of the activity on the vertical axis

 Knowt
Knowt
