
PY 131 Chapter 22: Electrostatics
Electrostatics
Phenomena associated with electricity and magnetism are found throughout nature
Lightning
Catfish / Electric eels
Lodestones
St Elmo’s fire
That all these phenomena are related was not always appreciated. Eventually, it was recognized that there are two different phenomena – electricity and magnetism – and then later still, the connection between them was found.
Nowadays we talk about electromagnetism or E&M.
It was known in antiquity that if you rub certain materials, such as amber, the material acquired the ability to influence other objects.
The Greek for amber is ‘electron’
Such materials were called ‘electrics’.
Electrics include materials such as amber, glass, and silk
Rubbing electrics charges the material.
The proper name for generating an electric charge this way is the triboelectric effect.
Later it was found that electrics came in two kinds that Benjamin Franklin called positive and negative.
Franklin thought charge was a fluid and that too much of the electric fluid produced a positive charge, too little produced a negative one.
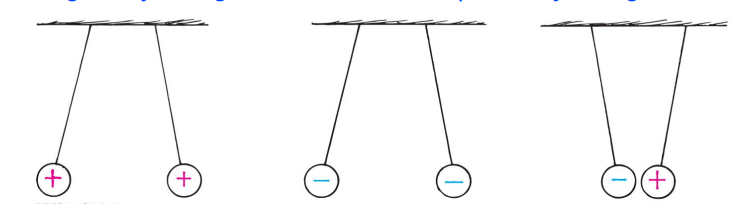
The two kinds of charges are such that:
Positively charged materials repel other positively charged materials,
Negatively charged materials repel other negatively charged materials,
Negatively charged materials attract positively charged materials,
The source of the charge on the electric materials was solved with the discovery of the particle we call electron. Electrons have negative charge so
Positively charged objects have a deficit of electrons.
Negatively charged objects have an excess of electrons.
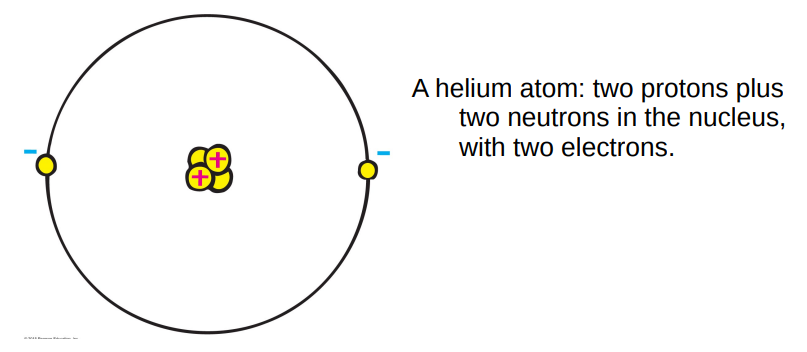
Later, after the acceptance of the existence of atoms, the positively charged part of the atom was found. Our modern picture is that:
atoms contain both positively charged particles (protons) concentrated in the middle of the atom (the nucleus) together with electrically neutral particles (the neutron).
Surrounding the nucleus are the negatively charged electrons.
The charge of the proton is exactly opposite that of the electron. The electrons are held to the nucleus by their opposite electric charges.
Charge
The SI unit of charge is the coulomb, with the symbol C.
Charge is ‘quantized’ - it comes in discrete lumps.
The charge of an electron is -1.6 x 10-19 C and this charge is usually given the symbol -e.
The smallest possible charge anything can have is ±e/3 but materials have charges which must be integers of e.
From experiment, it is found that electric charge is conserved. Electrons (rarely protons) can be moved from one material to another but the total number remains unchanged.
Electric charge is like energy or momentum.
In 1784 Coulomb measured the force between charged materials using a similar device to the one used by Cavendish to measure the force due to gravity.
Coulomb showed that the force between electric charges was very similar to gravity:
Like gravity, the size of the force was inversely proportional to the square of the distance between the charged materials.
Like gravity, the size of the force was proportional to the product of the charges of the two objects.
If the two charges are q1 and q2, and the distance between them is d, Coulomb’s Law is that the force F is given by
F=k (q_1 q_2 / d^2)
k is the constant of proportionality and in SI units is equal to k = 9 x 109 N m2 /C2
k is like Big G but much much bigger.
Conductors and Insulators
The materials that could be charged by rubbing were called electrics.
Electrics include amber, glass, PVC, and silk
The materials that could not be charged by rubbing (or not charged much) were known as non-electrics.
Non-electrics include all metals and water.
Non-electrics can be charged by the method of induction.
Nowadays we call electrics (electrical) insulators and nonelectrics (electrical) conductors. In a conductor the electrons flow easily through the material, in an insulator, they flow very poorly.
The ability of electrons to flow through a material is called the material’s conductivity.
The inverse of conductivity is known as resistivity.
The best conductors are plasmas which have free electrons
the positively charged ions are much heavier and don’t move as much.
The best metallic conductors are silver, copper, and gold.
Good insulators include glass, diamond, air, and plastics.
There is a class of materials, called semiconductors, which are not good insulators but also not good conductors.
Examples include silicon and germanium.
The conductivity of metals drops as their temperature rises, for semiconductors it rises.
As electrons flow through a material, they collide with the atoms and heat the material.
For some materials (including many metals) below a certain temperature the resistivity vanishes so there is no heating as the electrons flow through the material
Materials with zero resistivity are called superconductors.
If a flow of electrons is established in a superconductor, the flow will continue forever (or until the material stops being a superconductor).
The first superconductor was mercury below 4 K.
Induction
Conductors cannot be charged (or charged much) by rubbing because any electrons the conductor loses/acquires are quickly regained/lost.
But if a conductor is isolated so that excess electrons cannot easily leave or an electron deficit easily refilled, a conductor can be charged by rubbing e.g. aircraft can acquire a charge in flight due to friction with the air.
A much better way to put a large charge on a conductor is by the method of induction.
The conductor we wish to charge is placed in contact with another conductor (e.g. the Earth).
An object which is already charged is brought close to the conductor we wish to charge.
Electrons either flow onto or away from the conductor we wish to charge depending on the charge of the object we have brought nearby.
The conductor we wish to charge is then disconnected from the second conductor.
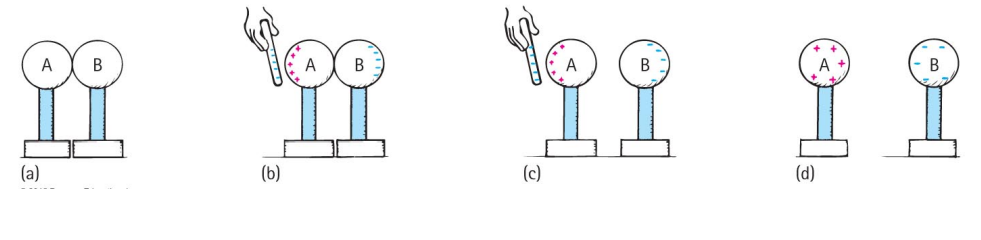
Charging by induction occurs during thunderstorms.
The bottom of the storm cloud is negatively charged.
This charge induces a positive charge in objects on the ground.
If the charges become sufficiently large, the negative charge on the cloud will flow to the positive charge on the ground as lightning.

Polarization
For all atoms, the charge center for the electrons coincides with the nucleus.
The charge center is the average position of the electrons.
But if a charged atom (ion) is nearby, the charge center for the electrons shifts and the atom becomes polarized.
The atom is still electrically neutral
This arrangement of a positive and negative charge of equal size separated by some distance is called a dipole.
Some molecules, e.g. water, are naturally polarized
Polarization of atoms/molecules in a material plus the distance dependence in Coulomb’s Law means a charge object can attract other objects

The plastic comb has a positive charge. When it is brought close to the piece of paper, it polarizes the paper.
The negative polarization charge is closest to the comb, the positive polarization charge is farther away.
The force on the paper due to the attraction of the negative charge to the comb is greater than the force on the paper due to repulsion of the positive charge.
The paper moves towards the comb.
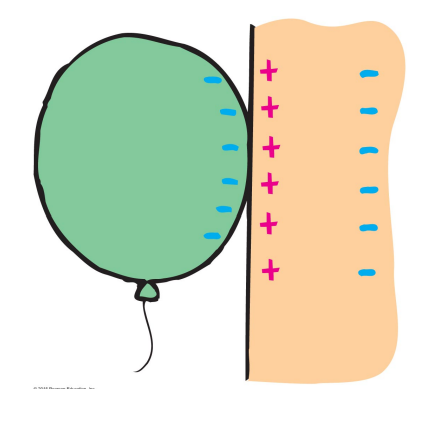
Polarization also explains why a rubbed balloon can stick to a wall.
Rubbing the balloon charges the balloon
Placing it close to a wall polarizes the molecules in the wall.
The attractive Coulomb force between the charged balloon and polarized molecules is larger than the repulsive Coulomb force between the balloon and polarized molecules
Electric Field
The modern way of thinking about the Coulomb force is that a charge fills the space around itself with an electric field.
Another charge (a test charge) placed in the field will experience a force from the charge that created the field.
The direction of the force on the test charge depends upon the charge which creates the field.
Field lines indicate the direction a positive test charge will move if placed at that location.

For an electric dipole the field looks like:

Since the electrons in a conductor are free to move, there can be no electric field inside the conductor.
If there was a field, the electrons would move along the field lines until they can move no further.
If a conductor is charged, all the charge resides on the outside of the conductor.
Surrounding an object with a conductor shields the object from electric fields.
The safest place to be in a thunderstorm is in a car.
Shielding of electric fields is why your cell phone usually doesn’t work inside an elevator.
Enclosures which shield objects from electric fields are called Faraday Cages.
Electric Potential
The Coulomb force is a conservative force which means it has a potential energy.
The change in the electric potential energy is the negative of the work done by the Coulomb force as the distance between two charges is changed.
So if the charges go around each other in a circle there is no work done.
Consider two positive charges which are brought closer together.
The Coulomb force does negative work → the electric potential energy of the charges increases.

If instead, you move the charges apart, the Coulomb force does positive work and the potential energy decreases
If one of the charges changes sign, the direction of the Coulomb force is reversed:
now moving the charges together decreases the electric potential energy.
moving the charges apart increases the electric potential energy
The potential energy of a test charge of 1 coulomb at a given location is called the potential of the field at that location
The SI unit of potential is the volt, the symbol is V.
The potential of a field is sometimes called the voltage.
Capacitors
A capacitor is a device which stores electrical potential energy.
The simplest capacitor is two parallel conducting plates.
One plate has a charge +Q, the other has a charge -Q
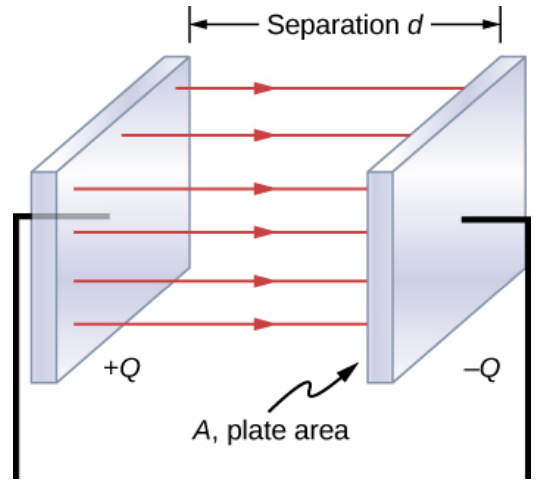
There is a voltage difference V between the two plates.
The closer the plates the larger the voltage difference
The energy stored in the capacitor is
Energy=Charge×Voltage
PY 131 Chapter 22: Electrostatics
Electrostatics
Phenomena associated with electricity and magnetism are found throughout nature
Lightning
Catfish / Electric eels
Lodestones
St Elmo’s fire
That all these phenomena are related was not always appreciated. Eventually, it was recognized that there are two different phenomena – electricity and magnetism – and then later still, the connection between them was found.
Nowadays we talk about electromagnetism or E&M.
It was known in antiquity that if you rub certain materials, such as amber, the material acquired the ability to influence other objects.
The Greek for amber is ‘electron’
Such materials were called ‘electrics’.
Electrics include materials such as amber, glass, and silk
Rubbing electrics charges the material.
The proper name for generating an electric charge this way is the triboelectric effect.
Later it was found that electrics came in two kinds that Benjamin Franklin called positive and negative.
Franklin thought charge was a fluid and that too much of the electric fluid produced a positive charge, too little produced a negative one.
The two kinds of charges are such that:
Positively charged materials repel other positively charged materials,
Negatively charged materials repel other negatively charged materials,
Negatively charged materials attract positively charged materials,

The source of the charge on the electric materials was solved with the discovery of the particle we call electron. Electrons have negative charge so
Positively charged objects have a deficit of electrons.
Negatively charged objects have an excess of electrons.
Later, after the acceptance of the existence of atoms, the positively charged part of the atom was found. Our modern picture is that:
atoms contain both positively charged particles (protons) concentrated in the middle of the atom (the nucleus) together with electrically neutral particles (the neutron).
Surrounding the nucleus are the negatively charged electrons.
The charge of the proton is exactly opposite that of the electron. The electrons are held to the nucleus by their opposite electric charges.

Charge
The SI unit of charge is the coulomb, with the symbol C.
Charge is ‘quantized’ - it comes in discrete lumps.
The charge of an electron is -1.6 x 10-19 C and this charge is usually given the symbol -e.
The smallest possible charge anything can have is ±e/3 but materials have charges which must be integers of e.
From experiment, it is found that electric charge is conserved. Electrons (rarely protons) can be moved from one material to another but the total number remains unchanged.
Electric charge is like energy or momentum.
In 1784 Coulomb measured the force between charged materials using a similar device to the one used by Cavendish to measure the force due to gravity.
Coulomb showed that the force between electric charges was very similar to gravity:
Like gravity, the size of the force was inversely proportional to the square of the distance between the charged materials.
Like gravity, the size of the force was proportional to the product of the charges of the two objects.
If the two charges are q1 and q2, and the distance between them is d, Coulomb’s Law is that the force F is given by
F=k (q_1 q_2 / d^2)
k is the constant of proportionality and in SI units is equal to k = 9 x 109 N m2 /C2
k is like Big G but much much bigger.
Conductors and Insulators
The materials that could be charged by rubbing were called electrics.
Electrics include amber, glass, PVC, and silk
The materials that could not be charged by rubbing (or not charged much) were known as non-electrics.
Non-electrics include all metals and water.
Non-electrics can be charged by the method of induction.
Nowadays we call electrics (electrical) insulators and nonelectrics (electrical) conductors. In a conductor the electrons flow easily through the material, in an insulator, they flow very poorly.
The ability of electrons to flow through a material is called the material’s conductivity.
The inverse of conductivity is known as resistivity.
The best conductors are plasmas which have free electrons
the positively charged ions are much heavier and don’t move as much.
The best metallic conductors are silver, copper, and gold.
Good insulators include glass, diamond, air, and plastics.
There is a class of materials, called semiconductors, which are not good insulators but also not good conductors.
Examples include silicon and germanium.
The conductivity of metals drops as their temperature rises, for semiconductors it rises.
As electrons flow through a material, they collide with the atoms and heat the material.
For some materials (including many metals) below a certain temperature the resistivity vanishes so there is no heating as the electrons flow through the material
Materials with zero resistivity are called superconductors.
If a flow of electrons is established in a superconductor, the flow will continue forever (or until the material stops being a superconductor).
The first superconductor was mercury below 4 K.
Induction
Conductors cannot be charged (or charged much) by rubbing because any electrons the conductor loses/acquires are quickly regained/lost.
But if a conductor is isolated so that excess electrons cannot easily leave or an electron deficit easily refilled, a conductor can be charged by rubbing e.g. aircraft can acquire a charge in flight due to friction with the air.
A much better way to put a large charge on a conductor is by the method of induction.
The conductor we wish to charge is placed in contact with another conductor (e.g. the Earth).
An object which is already charged is brought close to the conductor we wish to charge.
Electrons either flow onto or away from the conductor we wish to charge depending on the charge of the object we have brought nearby.
The conductor we wish to charge is then disconnected from the second conductor.
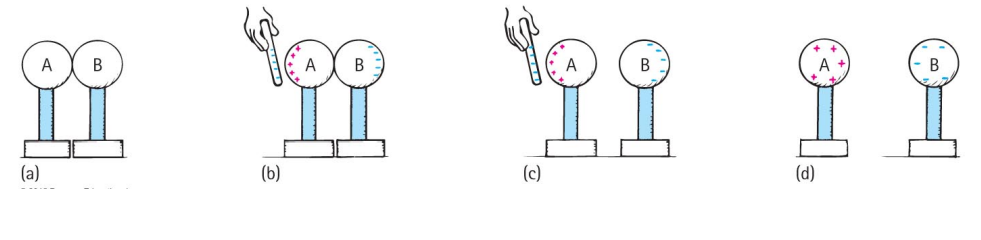
Charging by induction occurs during thunderstorms.
The bottom of the storm cloud is negatively charged.
This charge induces a positive charge in objects on the ground.
If the charges become sufficiently large, the negative charge on the cloud will flow to the positive charge on the ground as lightning.

Polarization
For all atoms, the charge center for the electrons coincides with the nucleus.
The charge center is the average position of the electrons.
But if a charged atom (ion) is nearby, the charge center for the electrons shifts and the atom becomes polarized.
The atom is still electrically neutral
This arrangement of a positive and negative charge of equal size separated by some distance is called a dipole.
Some molecules, e.g. water, are naturally polarized
Polarization of atoms/molecules in a material plus the distance dependence in Coulomb’s Law means a charge object can attract other objects

The plastic comb has a positive charge. When it is brought close to the piece of paper, it polarizes the paper.
The negative polarization charge is closest to the comb, the positive polarization charge is farther away.
The force on the paper due to the attraction of the negative charge to the comb is greater than the force on the paper due to repulsion of the positive charge.
The paper moves towards the comb.
Polarization also explains why a rubbed balloon can stick to a wall.
Rubbing the balloon charges the balloon
Placing it close to a wall polarizes the molecules in the wall.
The attractive Coulomb force between the charged balloon and polarized molecules is larger than the repulsive Coulomb force between the balloon and polarized molecules

Electric Field
The modern way of thinking about the Coulomb force is that a charge fills the space around itself with an electric field.
Another charge (a test charge) placed in the field will experience a force from the charge that created the field.
The direction of the force on the test charge depends upon the charge which creates the field.
Field lines indicate the direction a positive test charge will move if placed at that location.

For an electric dipole the field looks like:

Since the electrons in a conductor are free to move, there can be no electric field inside the conductor.
If there was a field, the electrons would move along the field lines until they can move no further.
If a conductor is charged, all the charge resides on the outside of the conductor.
Surrounding an object with a conductor shields the object from electric fields.
The safest place to be in a thunderstorm is in a car.
Shielding of electric fields is why your cell phone usually doesn’t work inside an elevator.
Enclosures which shield objects from electric fields are called Faraday Cages.
Electric Potential
The Coulomb force is a conservative force which means it has a potential energy.
The change in the electric potential energy is the negative of the work done by the Coulomb force as the distance between two charges is changed.
So if the charges go around each other in a circle there is no work done.
Consider two positive charges which are brought closer together.
The Coulomb force does negative work → the electric potential energy of the charges increases.

If instead, you move the charges apart, the Coulomb force does positive work and the potential energy decreases
If one of the charges changes sign, the direction of the Coulomb force is reversed:
now moving the charges together decreases the electric potential energy.
moving the charges apart increases the electric potential energy
The potential energy of a test charge of 1 coulomb at a given location is called the potential of the field at that location
The SI unit of potential is the volt, the symbol is V.
The potential of a field is sometimes called the voltage.
Capacitors
A capacitor is a device which stores electrical potential energy.
The simplest capacitor is two parallel conducting plates.
One plate has a charge +Q, the other has a charge -Q

There is a voltage difference V between the two plates.
The closer the plates the larger the voltage difference
The energy stored in the capacitor is
Energy=Charge×Voltage
 Knowt
Knowt
