
Quater 3 - Periodic Table- TRENDS
DO NOT CLICK FLASHCARDS FROM HERE (OR STUDY). Click Here
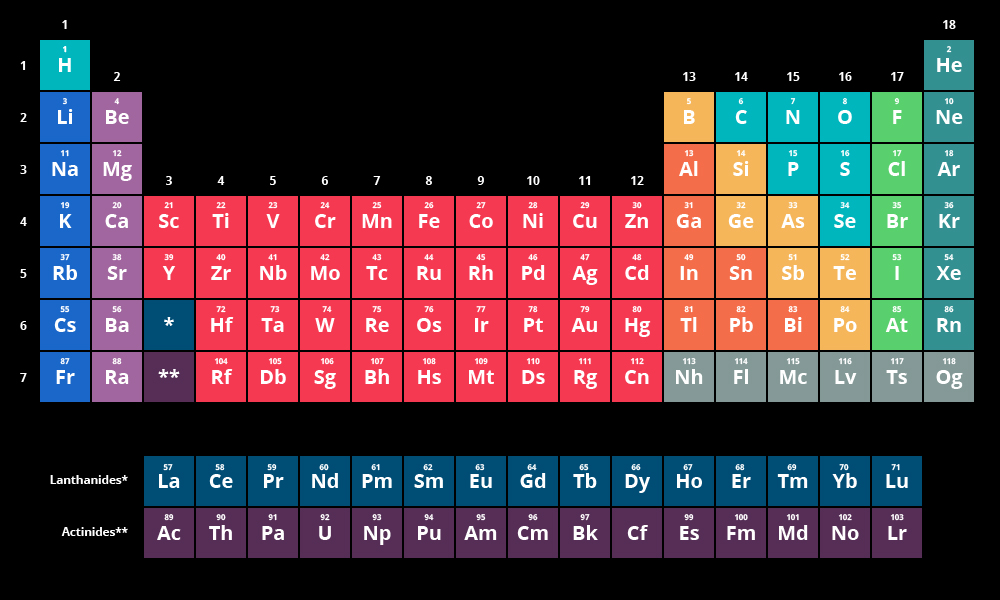
Periodic Table Basics:
Ordered by Atomic Number (# of Protons)
Columns are groups (Tend to have the same valence electrons)
Rows are periods (Same amount of orbitals)
Main Groups: (Divided by staircase on the right hand side. In yellow in the picture above)
Metals- On the left side of the staircase.
Reactive
Ductile and Malleable
Usually good conductors
Usually solid at room temperature
Nonmetals- On the right of the staircase.
Brittle (Will break easily)
Not always solid at room temperature
Some of them are diatomic (Exist in pairs of atoms).
Do not conduct electricity.
Metalloids- Touching the staircase. (Yellow elements above)
Can act like either a metal or a nonmetal depending on the conditions.
Other Groups: (Location on picture in parentheses)
Alkaline: (Dark blue on left)
Group 1 (1 valence electron)
Very reactive with water
Very soft
Alkaline Earth: (Light purple on left)
Group 2 (2 valence electrons)
Also reactive with water
Also soft
Transition Metals: (Red in the middle)
Groups 3-12 (Random amounts of valence electrons)
Traditional metals like Copper
Good conductors
Hard
Nonmetals: (Turquoise next to the yellow staircase)
Groups 13-16 (3-6 valence electrons)
Same characteristics as above for nonmetals
Can be named more specifically by the element at the top of the group
Halogens: (Lime green on the far right)
Group 17 (7 valence electrons)
Gasses
Diatomic
Noble Gasses: (Dark turquoise on the right end)
Group 18 (8 valence electrons)
Stable (Non-reactive)
Gasses
Periodic Trends:
Atomic Radius:
Radius of an atom. Distance from the nucleus to its farthest orbital
Increases from Right to left, and top to bottom on the table.
Francinium (Fr) has the largest Atomic Radius.
Electronegativity:
Attraction of an atom to electrons
Atoms will even attract its own electrons, pulling its orbitals farther in.
Decreseres Atomic Radius
Increases from left to right, and bottom to top on the table.
Does not affect Group 18
Categorized by a number 1-4 (4 being the strongest attraction)
Fluorine (F) has the most attraction (4)
Ionization energy:
The amount of energy for an atom to take another atom’s electrons. To become an Ion.
Atoms will attract not just it’s own electrons, but other atom’s electrons.
Most atoms want 8 valence electrons so that's what they want to pull.
Increases from left to right, and bottom to top on the table.
Directly related to Electronegativity.
Also does not affect group 18.
Fluorine(F) is as
Elements on the far left just easily give up electrons, rather than taking them.
Next Unit: Unit 4- Bonding
Quater 3 - Periodic Table- TRENDS
DO NOT CLICK FLASHCARDS FROM HERE (OR STUDY). Click Here

Periodic Table Basics:
Ordered by Atomic Number (# of Protons)
Columns are groups (Tend to have the same valence electrons)
Rows are periods (Same amount of orbitals)
Main Groups: (Divided by staircase on the right hand side. In yellow in the picture above)
Metals- On the left side of the staircase.
Reactive
Ductile and Malleable
Usually good conductors
Usually solid at room temperature
Nonmetals- On the right of the staircase.
Brittle (Will break easily)
Not always solid at room temperature
Some of them are diatomic (Exist in pairs of atoms).
Do not conduct electricity.
Metalloids- Touching the staircase. (Yellow elements above)
Can act like either a metal or a nonmetal depending on the conditions.
Other Groups: (Location on picture in parentheses)
Alkaline: (Dark blue on left)
Group 1 (1 valence electron)
Very reactive with water
Very soft
Alkaline Earth: (Light purple on left)
Group 2 (2 valence electrons)
Also reactive with water
Also soft
Transition Metals: (Red in the middle)
Groups 3-12 (Random amounts of valence electrons)
Traditional metals like Copper
Good conductors
Hard
Nonmetals: (Turquoise next to the yellow staircase)
Groups 13-16 (3-6 valence electrons)
Same characteristics as above for nonmetals
Can be named more specifically by the element at the top of the group
Halogens: (Lime green on the far right)
Group 17 (7 valence electrons)
Gasses
Diatomic
Noble Gasses: (Dark turquoise on the right end)
Group 18 (8 valence electrons)
Stable (Non-reactive)
Gasses
Periodic Trends:
Atomic Radius:
Radius of an atom. Distance from the nucleus to its farthest orbital
Increases from Right to left, and top to bottom on the table.
Francinium (Fr) has the largest Atomic Radius.
Electronegativity:
Attraction of an atom to electrons
Atoms will even attract its own electrons, pulling its orbitals farther in.
Decreseres Atomic Radius
Increases from left to right, and bottom to top on the table.
Does not affect Group 18
Categorized by a number 1-4 (4 being the strongest attraction)
Fluorine (F) has the most attraction (4)
Ionization energy:
The amount of energy for an atom to take another atom’s electrons. To become an Ion.
Atoms will attract not just it’s own electrons, but other atom’s electrons.
Most atoms want 8 valence electrons so that's what they want to pull.
Increases from left to right, and bottom to top on the table.
Directly related to Electronegativity.
Also does not affect group 18.
Fluorine(F) is as
Elements on the far left just easily give up electrons, rather than taking them.
 Knowt
Knowt