
Chapter 2: Separation Techniques
2.1-Mixtures and Chromatography
Mixtures are easily separated-not like compounds
Unlike in a compound, there’s no chemical bond between the different parts of a mixture
The parts of a a mixture can be either elements or compounds, and they can be separated out by physical methods such as filtration, crystallisation, simple distillation, fractional distillation and chromatography
Air is a mixture of gases, mainly nitrogen, oxygen, carbon dioxide and argon
Crude oil: a mixture of different length hydrocarbon molecules
The properties of a mixture are just a mixture of the properties of the separate parts-the chemical properties of a substance aren’t affected by it being part of a mixture
For example, a mixture of iron powder and sulfur powder will show the properties of both iron and sulfur. It will contain grey magnetic bits of iron and bright yellow bits of sulfur.
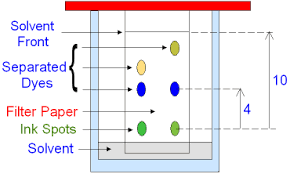
You need to know how to do paper chromatography
One method of separating substances in a mixture is through chromatography. This techniques can be used to separate different dyes in an ink. Here’s how you can do it:
Draw a line near the bottom of a sheet of filter paper.
Use a pencil to do this-pencil marks are insoluble and won’t dissolve in the solvent.
Add a spot of the ink to the line and place the sheet in a beaker of solvent, e.g. water.
The solvent used depends on what’s being tested. Some compounds dissolve well in water, but sometimes other solvents, like ethanol, are needed.
Make sure the ink isn’t touching the solvent-you don’t want it to dissolve into it
Place a lid on top of the container to stop the solvent evaporating
The solvent seeps up the paper, carrying the ink with it
Each different dye in the ink will move up the paper at a different rate so the dyes will separate out. Each dye will form a spot in a different place-1 spot per dye in the ink
If any of the dyes in the ink are insoluble(won’t dissolve) in the solvent you’ve used, they’ll stay on the baseline
When the solvent has nearly reached the top of the paper, take the paper out of the beaker and leave it to dry
The end result is a pattern of spots called chromatogram
2.2-More Separation Techniques
Filtration separates insoluble solids from liquids
Filtration can be used if your product is an insoluble solid that needs to be seperated from a liquid reaction mixture
It can be used in purification as well. For example, solid impurities in the reaction mixture can be separated out using filtration
Insoluble means the solid can’t be dissolved in the liquid
Two ways to separate soluble solids from solutions
If a solid can be dissolved it’s described as being soluble. There are two methods you can use to separate a soluble salt from a solution-evaporation and crystallisation
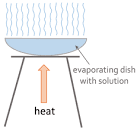
Evaporation
Pour the solution into an evaporating dish
Slowly heat the solution.
The solvent will evaport and the solution will get more concentrated. Eventually, crystals will start to form
Keep heating the evaporating dish until all you have left are dry crystals
Evaporation is a really quick way of separating a soluble salt from a solution, but you can only use it if the salt doesn’t decompose(break down) when its heated. Otherwise, you’ll have to use crystallisation
Crystallisation
Pour the solution into an evaporating dish and gently hear the solution. Some of the solvent will evaport and the solution will get more concentrated
Once some of the solvent has evaporated, or when you see crystals start to form(the point of crystallisation), remove the dish from the heat and leave the solution to cool
The salt should start to form crystals as it become insoluble in the cold, highly concentrated solution
Filter the crystals out of the solution, and leave them in a warm place to dry. You could also use a drying oven or a desiccator
Filtration and crystallisation can be used to separate rock salt
Roc salt is simply a mixture of salt and sand(they spread it on the roads in winter).
Salt and sand are both compounds-but salt dissolves in water and sand doesn’t. This vital difference in their physical properties gives a great way to separate them. Here’s what to do…
Grind the mixture to make sure the salt crystals are small, so will dissolve easily
Put the mixture in water and stir. The salt will dissolve, but the sand won’t.
You can heat the mixture to help dissolve the salt
Filter the mixture. The grains of sand won’t fit through the tiny holes in the filter paper, so they collect on the paper instead. The salt passes through the filter paper as it’s part of the solution
Evaporate the water from the salt so that it forms dry crystals
2.3-Distillation
Simple distillation is used to separate out solution
Simple distillation: used for separating out a liquid from a solution
The solution is heated. The part of the solution that has the lowest boiling point evaporates first
The vapour is then cooled, condenses and is collected
The rest of the solution is left behind in the flask
You can use simple distillation to get pure water from seawater. The water evaporates and is condenses and collected. Eventually you’ll end up with just the salt left in the flask
The problem with simple distillation is that you can only use it to separate thing with very different boiling points-if the temperature goes higher than the boiling point of the substance with the higher boiling point, they will mix again
Make sure the water goes in at the bottom of the condenser and out at the top
If you have a mixture of liquids with similar boiling points you need another method to separate them-like fractional distillation
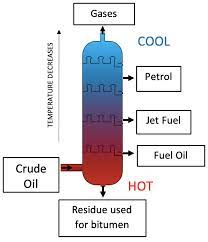
Fractional distillation is used to separate a mixture of liquids
If you’ve got a mixture of liquids you can separate it using fractional distillation. Here is a lab demonstration used to model fractional distillation of crude oil at a a refinery
You put your mixture in a flask and stick a fractionating column o top. Then you heat it.
The different liquids will all have different boiling points-so they will evaporate at different temperatures
The liquid with the lowest boiling point evaporates first. When the e temperature on the thermometer matches the boiling point of this liquid, it will react the top of the column
Liquids with higher boiling points might also start to evaporate. But the column is cooler towards the top. So they will only get part of the way up before condensing and running back down towards the flask
When the first liquid has been collected, you raise the temperature until the next one reaches the top
Chapter 2: Separation Techniques
2.1-Mixtures and Chromatography
Mixtures are easily separated-not like compounds
Unlike in a compound, there’s no chemical bond between the different parts of a mixture
The parts of a a mixture can be either elements or compounds, and they can be separated out by physical methods such as filtration, crystallisation, simple distillation, fractional distillation and chromatography
Air is a mixture of gases, mainly nitrogen, oxygen, carbon dioxide and argon
Crude oil: a mixture of different length hydrocarbon molecules
The properties of a mixture are just a mixture of the properties of the separate parts-the chemical properties of a substance aren’t affected by it being part of a mixture
For example, a mixture of iron powder and sulfur powder will show the properties of both iron and sulfur. It will contain grey magnetic bits of iron and bright yellow bits of sulfur.
You need to know how to do paper chromatography
One method of separating substances in a mixture is through chromatography. This techniques can be used to separate different dyes in an ink. Here’s how you can do it:
Draw a line near the bottom of a sheet of filter paper.
Use a pencil to do this-pencil marks are insoluble and won’t dissolve in the solvent.
Add a spot of the ink to the line and place the sheet in a beaker of solvent, e.g. water.
The solvent used depends on what’s being tested. Some compounds dissolve well in water, but sometimes other solvents, like ethanol, are needed.
Make sure the ink isn’t touching the solvent-you don’t want it to dissolve into it
Place a lid on top of the container to stop the solvent evaporating
The solvent seeps up the paper, carrying the ink with it
Each different dye in the ink will move up the paper at a different rate so the dyes will separate out. Each dye will form a spot in a different place-1 spot per dye in the ink
If any of the dyes in the ink are insoluble(won’t dissolve) in the solvent you’ve used, they’ll stay on the baseline
When the solvent has nearly reached the top of the paper, take the paper out of the beaker and leave it to dry
The end result is a pattern of spots called chromatogram

2.2-More Separation Techniques
Filtration separates insoluble solids from liquids
Filtration can be used if your product is an insoluble solid that needs to be seperated from a liquid reaction mixture
It can be used in purification as well. For example, solid impurities in the reaction mixture can be separated out using filtration
Insoluble means the solid can’t be dissolved in the liquid
Two ways to separate soluble solids from solutions
If a solid can be dissolved it’s described as being soluble. There are two methods you can use to separate a soluble salt from a solution-evaporation and crystallisation
Evaporation
Pour the solution into an evaporating dish
Slowly heat the solution.
The solvent will evaport and the solution will get more concentrated. Eventually, crystals will start to form
Keep heating the evaporating dish until all you have left are dry crystals
Evaporation is a really quick way of separating a soluble salt from a solution, but you can only use it if the salt doesn’t decompose(break down) when its heated. Otherwise, you’ll have to use crystallisation
Crystallisation
Pour the solution into an evaporating dish and gently hear the solution. Some of the solvent will evaport and the solution will get more concentrated
Once some of the solvent has evaporated, or when you see crystals start to form(the point of crystallisation), remove the dish from the heat and leave the solution to cool
The salt should start to form crystals as it become insoluble in the cold, highly concentrated solution
Filter the crystals out of the solution, and leave them in a warm place to dry. You could also use a drying oven or a desiccator
Filtration and crystallisation can be used to separate rock salt
Roc salt is simply a mixture of salt and sand(they spread it on the roads in winter).
Salt and sand are both compounds-but salt dissolves in water and sand doesn’t. This vital difference in their physical properties gives a great way to separate them. Here’s what to do…
Grind the mixture to make sure the salt crystals are small, so will dissolve easily
Put the mixture in water and stir. The salt will dissolve, but the sand won’t.
You can heat the mixture to help dissolve the salt
Filter the mixture. The grains of sand won’t fit through the tiny holes in the filter paper, so they collect on the paper instead. The salt passes through the filter paper as it’s part of the solution
Evaporate the water from the salt so that it forms dry crystals

2.3-Distillation
Simple distillation is used to separate out solution
Simple distillation: used for separating out a liquid from a solution
The solution is heated. The part of the solution that has the lowest boiling point evaporates first
The vapour is then cooled, condenses and is collected
The rest of the solution is left behind in the flask
You can use simple distillation to get pure water from seawater. The water evaporates and is condenses and collected. Eventually you’ll end up with just the salt left in the flask
The problem with simple distillation is that you can only use it to separate thing with very different boiling points-if the temperature goes higher than the boiling point of the substance with the higher boiling point, they will mix again
Make sure the water goes in at the bottom of the condenser and out at the top
If you have a mixture of liquids with similar boiling points you need another method to separate them-like fractional distillation
Fractional distillation is used to separate a mixture of liquids
If you’ve got a mixture of liquids you can separate it using fractional distillation. Here is a lab demonstration used to model fractional distillation of crude oil at a a refinery
You put your mixture in a flask and stick a fractionating column o top. Then you heat it.
The different liquids will all have different boiling points-so they will evaporate at different temperatures
The liquid with the lowest boiling point evaporates first. When the e temperature on the thermometer matches the boiling point of this liquid, it will react the top of the column
Liquids with higher boiling points might also start to evaporate. But the column is cooler towards the top. So they will only get part of the way up before condensing and running back down towards the flask
When the first liquid has been collected, you raise the temperature until the next one reaches the top

 Knowt
Knowt
