
Atoms, Molecules, Ions
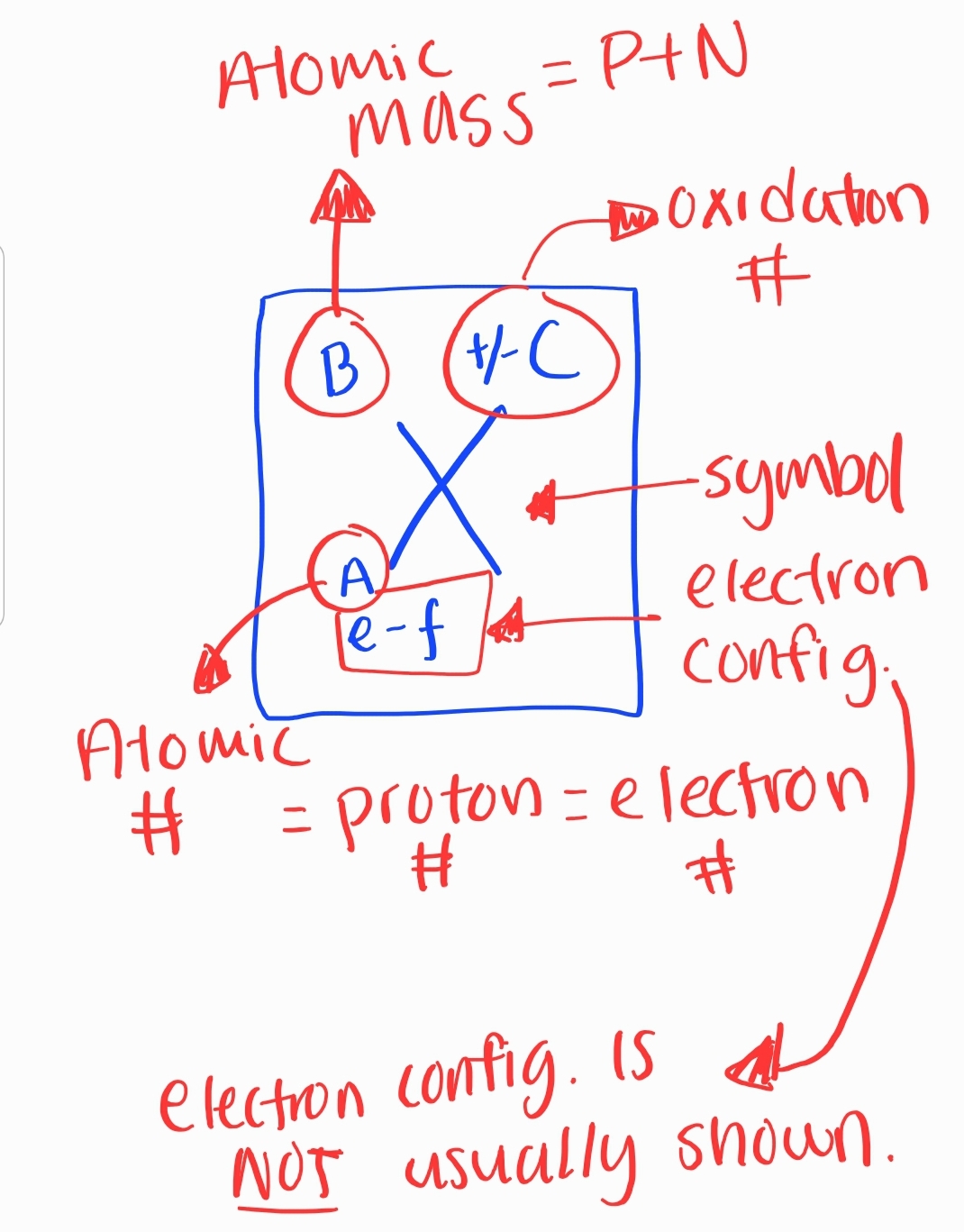
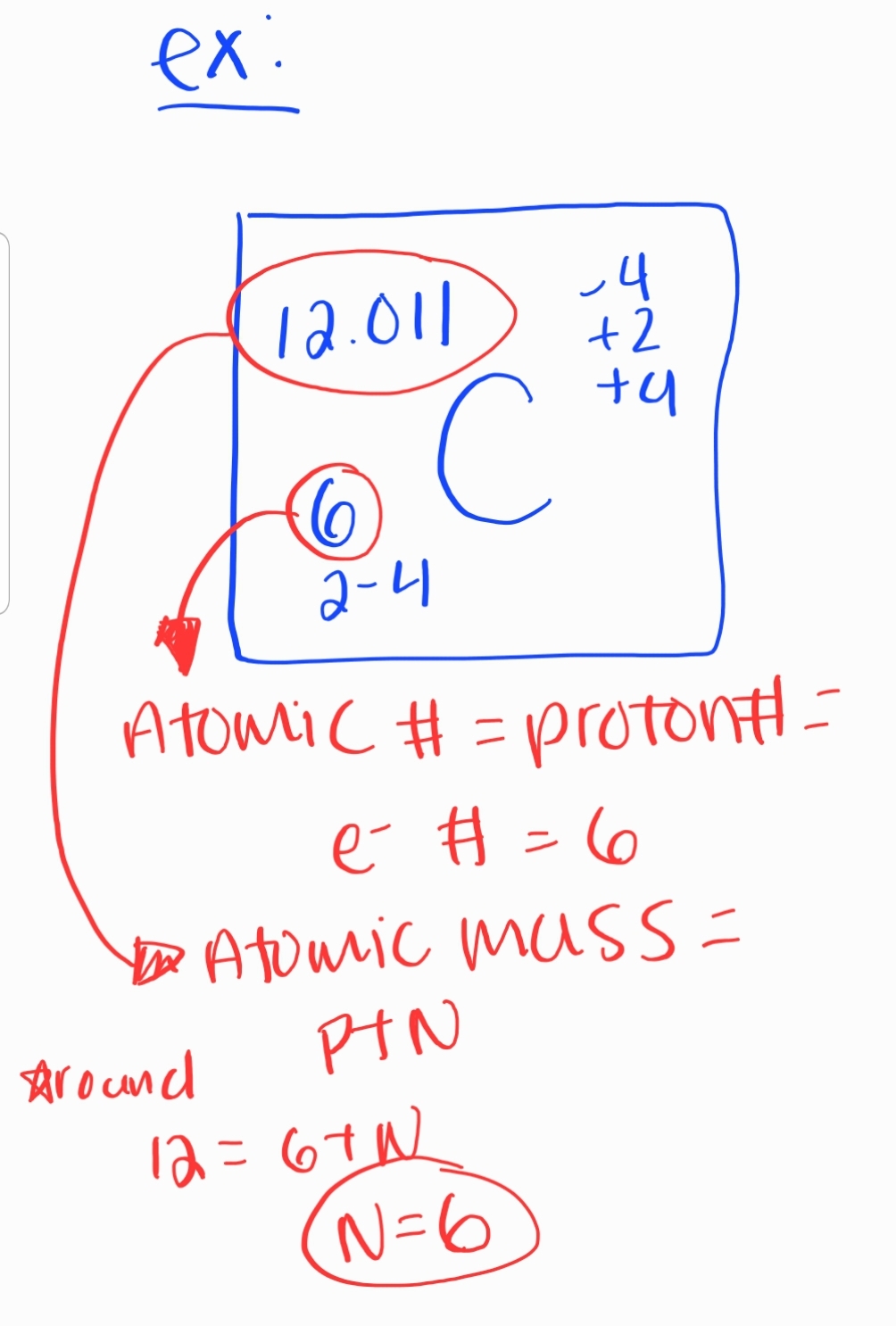
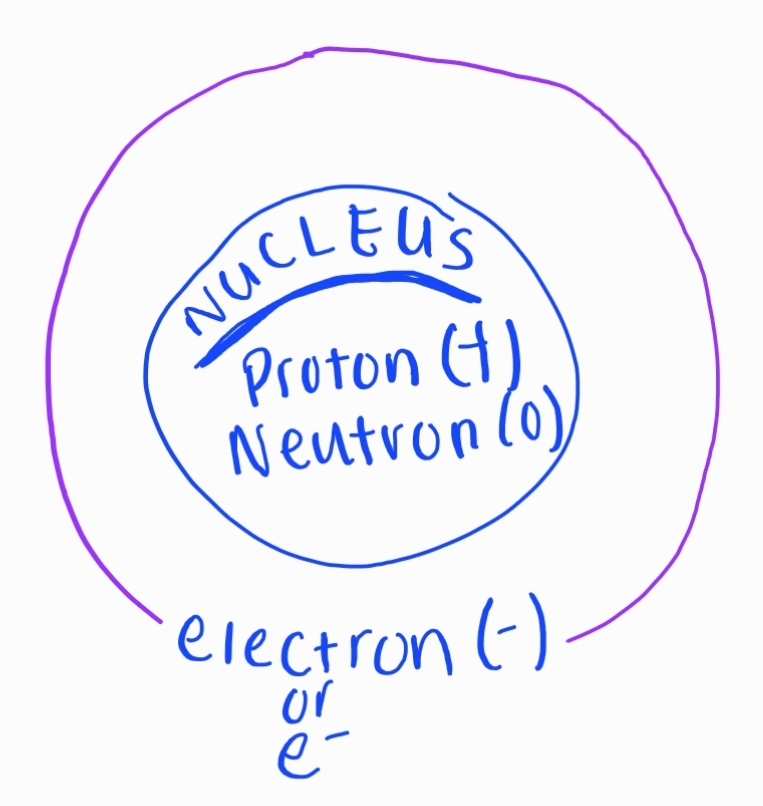
All atoms of an element must have the same ATOMIC #
Mind Map: Atoms, Molecules, Ions
Central Idea: Atoms
Definition: Basic building blocks of matter
Composed of: Protons, neutrons, and electrons
Main Branches
1. Atomic Structure
Protons
Positive charge
Located in the nucleus
Neutrons
No charge
Located in the nucleus
Electrons
Negative charge
Orbit around the nucleus
2. Elements
Definition: Pure substances made up of only one type of atom
Examples: Hydrogen, Oxygen, Carbon
3. Atomic Number and Mass
Atomic Number
Number of protons in an atom
Determines the element
Atomic Mass
Sum of protons and neutrons in an atom
Measured in atomic mass units (amu)
Sub-Branches
1. Molecules
Definition: Two or more atoms chemically bonded together
Types of Bonds
Covalent Bonds
Sharing of electrons between atoms
Examples: H2, O2, CO2
Ionic Bonds
Transfer of electrons between atoms
Examples: NaCl, MgO
2. Ions
Definition: Charged particles formed by gaining or losing electrons
Cations
Positively charged ions (more protons than electrons)
Formed by losing electrons
Anions
Negatively charged ions (more protons than electrons)
Formed by gaining electrons
Polyatomic Ion: electrically charged particle w 2+ atoms linked together so it behaves as a unit instead of separate atoms
charge belongs to the ion as a WHOLE
3. Compound
Definition: 2+ diff elements chemically bonded together
Examples: Water (H2O), Sodium Chloride (NaCl)
4. Chemical Reactions
Definition: Process where atoms are rearranged to form new substances
Reactants
Substances present before the reaction
Products
Substances formed after the reaction
5. Isotopes
Definition: Atoms of the same element with different numbers of neutrons
Ex: Carbon-12, Carbon-13, Carbon-14
6. Atomic Models
Dalton's Model
Thomson's Model
Rutherford's Model
Bohr's Model
Quantum Mechanical Model
7. Periodic Table
Organizes elements in an increasing atomic #
Atoms, Molecules, Ions



All atoms of an element must have the same ATOMIC #
Mind Map: Atoms, Molecules, Ions
Central Idea: Atoms
Definition: Basic building blocks of matter
Composed of: Protons, neutrons, and electrons
Main Branches
1. Atomic Structure
Protons
Positive charge
Located in the nucleus
Neutrons
No charge
Located in the nucleus
Electrons
Negative charge
Orbit around the nucleus
2. Elements
Definition: Pure substances made up of only one type of atom
Examples: Hydrogen, Oxygen, Carbon
3. Atomic Number and Mass
Atomic Number
Number of protons in an atom
Determines the element
Atomic Mass
Sum of protons and neutrons in an atom
Measured in atomic mass units (amu)
Sub-Branches
1. Molecules
Definition: Two or more atoms chemically bonded together
Types of Bonds
Covalent Bonds
Sharing of electrons between atoms
Examples: H2, O2, CO2
Ionic Bonds
Transfer of electrons between atoms
Examples: NaCl, MgO
2. Ions
Definition: Charged particles formed by gaining or losing electrons
Cations
Positively charged ions (more protons than electrons)
Formed by losing electrons
Anions
Negatively charged ions (more protons than electrons)
Formed by gaining electrons
Polyatomic Ion: electrically charged particle w 2+ atoms linked together so it behaves as a unit instead of separate atoms
charge belongs to the ion as a WHOLE
3. Compound
Definition: 2+ diff elements chemically bonded together
Examples: Water (H2O), Sodium Chloride (NaCl)
4. Chemical Reactions
Definition: Process where atoms are rearranged to form new substances
Reactants
Substances present before the reaction
Products
Substances formed after the reaction
5. Isotopes
Definition: Atoms of the same element with different numbers of neutrons
Ex: Carbon-12, Carbon-13, Carbon-14
6. Atomic Models
Dalton's Model
Thomson's Model
Rutherford's Model
Bohr's Model
Quantum Mechanical Model
7. Periodic Table
Organizes elements in an increasing atomic #
 Knowt
Knowt