
AP Biology Unit 1- The Chemistry Of Life Review
Topic 1.1: Structure of water and hydrogen bonding
Properties of Water
Hydrogen Bonds + Polarity
Polar: Oxygen has a partial negative charge, hydrogen has a positive charge
Hydrogen bond: Weak bond between hydrogen in a polar molecule and a small electronegative atom, forms due to the two atoms’ opposite charges
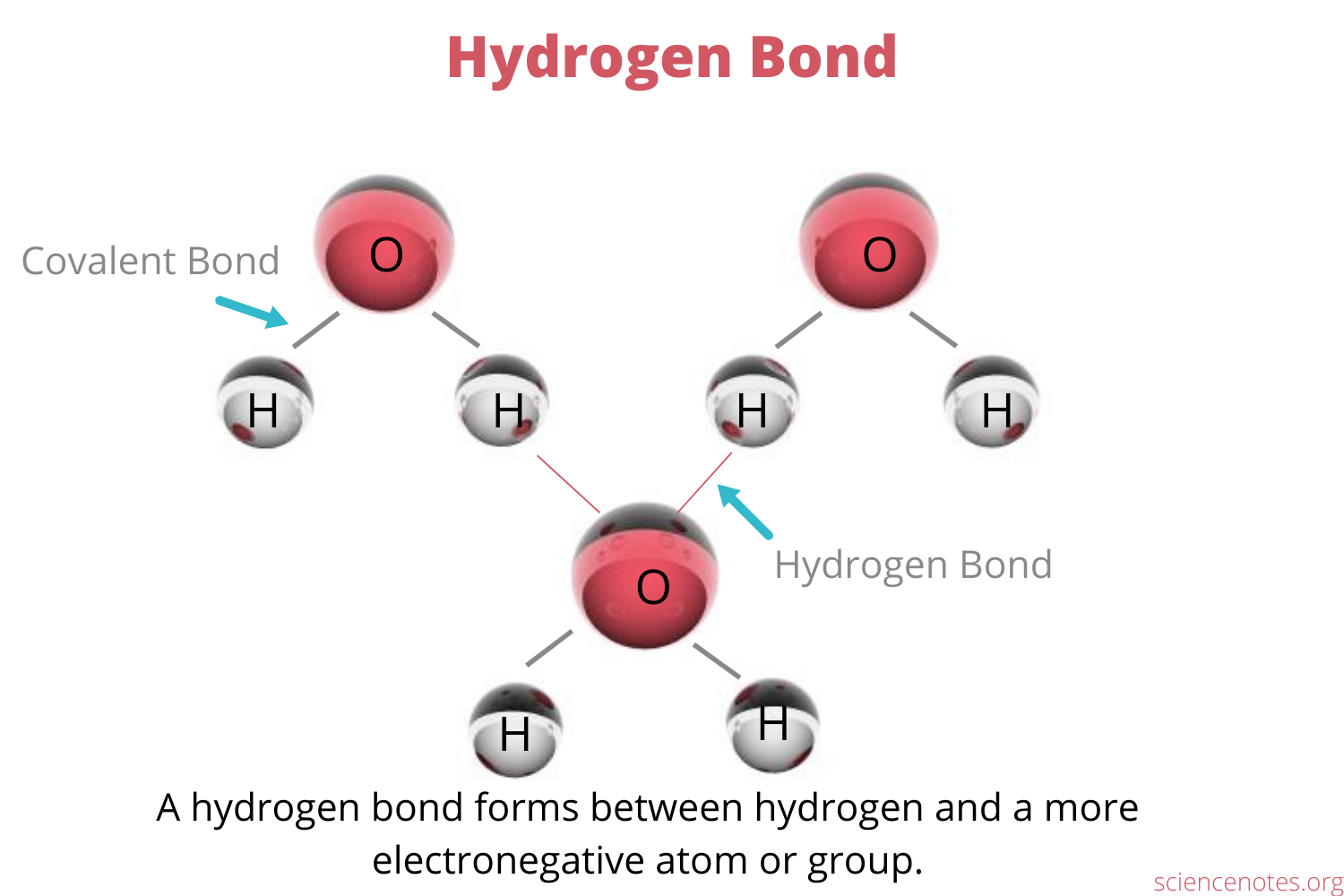
Cohesion: Hydrogen bonds hold a substance together
Adhesion: Clinging of one substance to another-- especially between water and another polar substance
Both adhesion and cohesion allow water to move against gravity
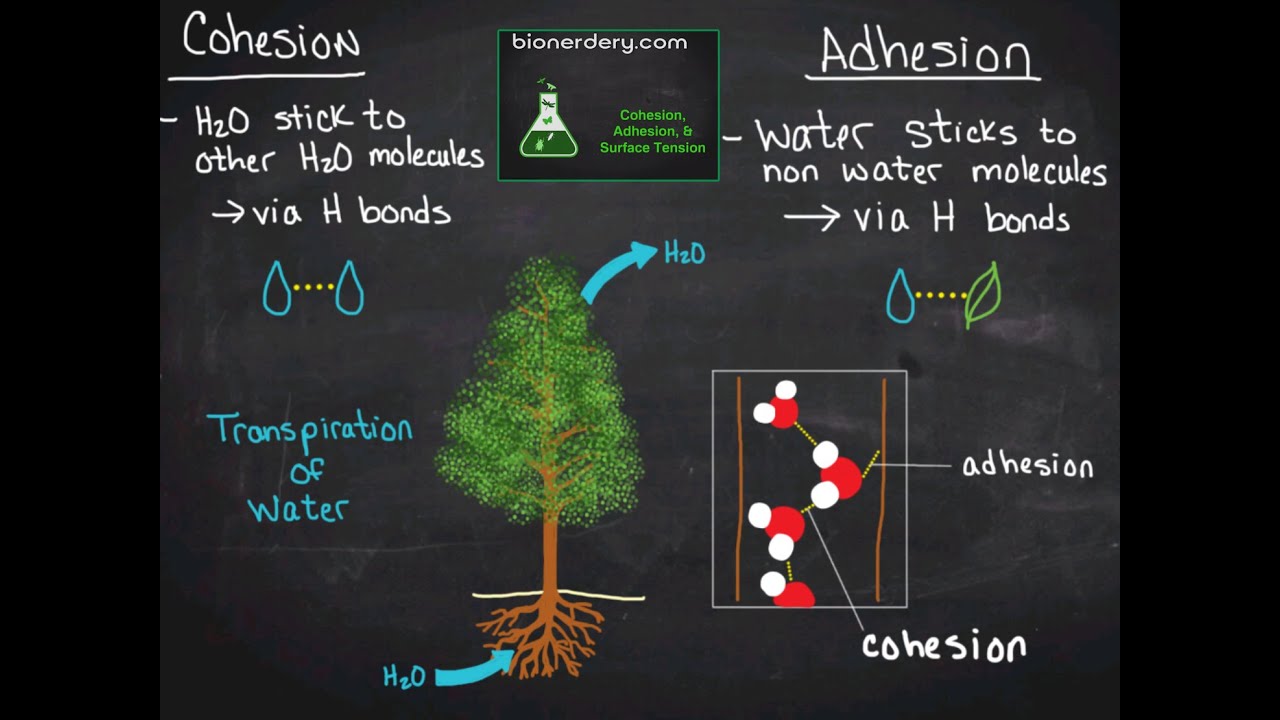
Surface temperature: How difficult it is to break or stretch the surface of a liquid, water has a high surface tension due to hydrogen bonds
Temperature Moderation
High specific heat: Water is able to absorb/release a lot of heat without its own temperature changing much, water absorbs warm air and releases it to cooler air. Allows climate moderation
High heat of vaporization: Water has to absorb a lot of heat to change from a liquid to a gas and water cools when its molecules evaporate. Allows evaporative cooling
Ice Floats
Water expands (becomes less dense) in its solid form, so ice floats
Floating ice insulates water below it

Solvent
Solvent: The substance that dissolves other molecules in a solution
H2O is a versatile solvent due to its polarity
Water can carry many chemicals, nutrients, and minerals throughout an ecosystem and the organisms within it
Topic 1.2-1.6: Macromolecules and the elements of life
Carbon
Carbon: Has 4 valence electrons so can form 4 covalent bonds, allowing it to be the backbone for many complex and diverse molecules
Organic compounds: Compounds containing carbon
Functional Groups (not required course content but definitely helpful to know)
Functional groups change function by being directly involved in chemical reactions
Hydroxyl (OH-): Alcohol, make substance more basic (raises pH)
Carboxyl (-COOH): Acid, contributes H+ (lowers pH)
Amino group (-NH2)
Phosphate group: Add negative charge to a molecule
Polymers and Monomers
Polymer: A molecule made of similar building blocks
Monomer: One of the units making up a polymer
Dehydration reaction: Monomers connected and covalently bonded by removing a hydrogen from both molecules and an oxygen from one (making H2O)
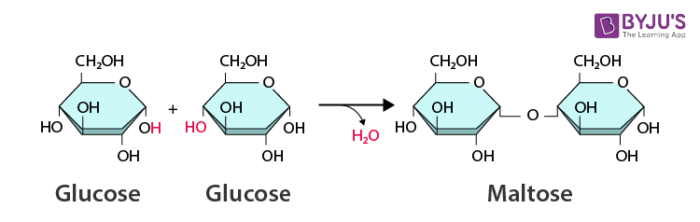
Hydrolysis: Bond between monomers of a polymer is broken by adding H2O
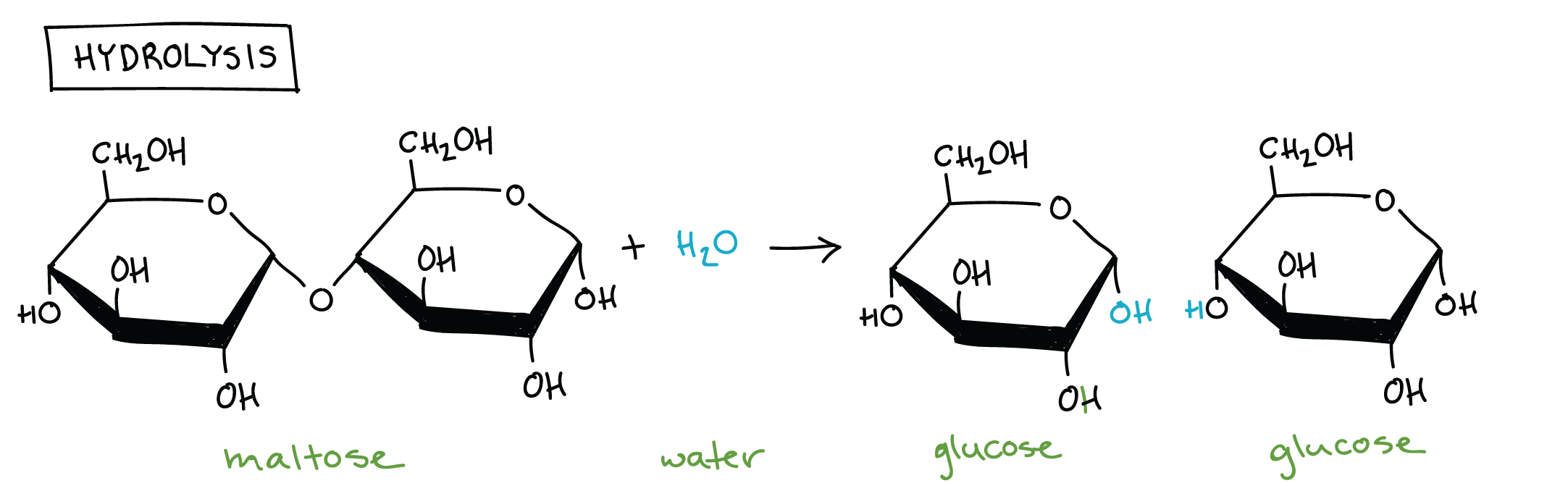
Carbohydrates
Contain elements: Carbon, hydrogen, and oxygen
Monomer: Monosaccharide
Polymer: Polysaccharides (chain of monosaccharides linked by covalent bonds called glycosidic linkages)
Functions: Provide and store energy, provide structural support
Important polysaccharides (all are glucose polymers)
Starch: Stores glucose in plants
Glycogen: Glucose storage in animals
Chitin: Structural polysaccharide in fungi cell wells and arthropod exoskeletons
Cellulose: Cell wall component for plant cells
Lipids
Elements: Carbon, hydrogen, oxygen (sometimes N or P)
No true monomers of lipids
Have little to no affinity for water (hydrophobic)
Function: Energy storage and structure
Saturated: No double bonds between carbon atoms, atoms tightly packed
Unsaturated: Double bonds between some carbons creating a kink in the chain

Trigylcerol: 3 fatty acids attached to one glycerol, energy storage
Phospholipid: Form phospholipid bilayer, have a hydrophilic head and hydrophobic tail
Cholesterol: Steroid that contributes to cell membrane integrity

Nucleic Acids
Elements: Carbon, hydrogen, oxygen, phosphorus
Monomers: Nucleotides, consist of a 5 carbon sugar, a phosphate, and a nitrogenous base
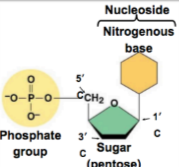
Polymers: Nucelic acids, linear sequences of nucleotides
Nitrogenous base pairs: Adenine pairs with thymine (or uracil in RNA), and cytosine pairs with guanine
Purines: Have a 2 ring structure (A and G)
Pyrmidines: Have a 1 ring structure ( C and T/U)
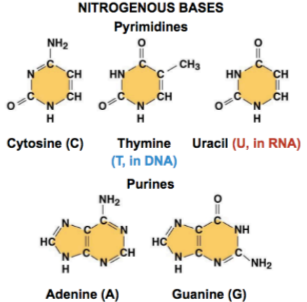
C and G are joined by 3 bonds, A and T are joined by 2
Directionality: Determined by orientation of sugars (hydroxyl = 3´ and phosphate = 5´)
Base pairs are joined by hydrogen bonds, nucleotides joined by covalent bonds
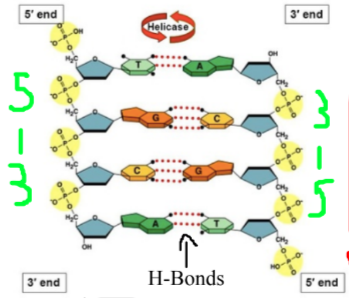
DNA: Antiparallel double helix (2 strands running in opposite directions), has deoxyribose
RNA: Single stranded, has ribose
Proteins
Elements: Carbon, hydrogen, oxygen, nitrogen, sulfur
Monomers: Amino acids, consist of carboxyl group + amino group + R group
Different amino acids have different R groups
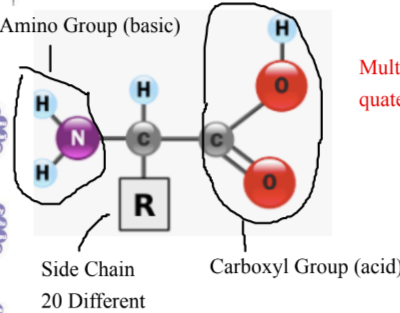
Polymer: Polypeptide
STRUCTURE
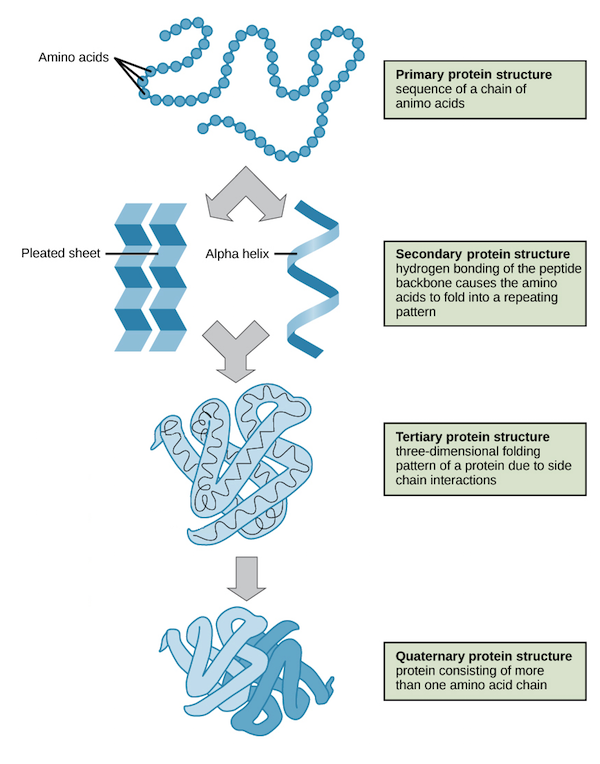
Primary: Linear sequence of covalently bonded amino acids
Secondary: Structure formed by interactions between polypeptide´s ¨Backbone¨ ( carboxyl and amino groups)
Tertiary: 3d structure formed by interactions ( hydrogen bonds, ionic bonds, disulfide bridge, hydrophobic, etc.) between R groups
Quaternary: More than one polypeptide chain joined together
AP Biology Unit 1- The Chemistry Of Life Review
Topic 1.1: Structure of water and hydrogen bonding
Properties of Water
Hydrogen Bonds + Polarity
Polar: Oxygen has a partial negative charge, hydrogen has a positive charge
Hydrogen bond: Weak bond between hydrogen in a polar molecule and a small electronegative atom, forms due to the two atoms’ opposite charges

Cohesion: Hydrogen bonds hold a substance together
Adhesion: Clinging of one substance to another-- especially between water and another polar substance
Both adhesion and cohesion allow water to move against gravity

Surface temperature: How difficult it is to break or stretch the surface of a liquid, water has a high surface tension due to hydrogen bonds
Temperature Moderation
High specific heat: Water is able to absorb/release a lot of heat without its own temperature changing much, water absorbs warm air and releases it to cooler air. Allows climate moderation
High heat of vaporization: Water has to absorb a lot of heat to change from a liquid to a gas and water cools when its molecules evaporate. Allows evaporative cooling
Ice Floats
Water expands (becomes less dense) in its solid form, so ice floats
Floating ice insulates water below it

Solvent
Solvent: The substance that dissolves other molecules in a solution
H2O is a versatile solvent due to its polarity
Water can carry many chemicals, nutrients, and minerals throughout an ecosystem and the organisms within it
Topic 1.2-1.6: Macromolecules and the elements of life
Carbon
Carbon: Has 4 valence electrons so can form 4 covalent bonds, allowing it to be the backbone for many complex and diverse molecules
Organic compounds: Compounds containing carbon

Functional Groups (not required course content but definitely helpful to know)
Functional groups change function by being directly involved in chemical reactions
Hydroxyl (OH-): Alcohol, make substance more basic (raises pH)
Carboxyl (-COOH): Acid, contributes H+ (lowers pH)
Amino group (-NH2)
Phosphate group: Add negative charge to a molecule
Polymers and Monomers
Polymer: A molecule made of similar building blocks
Monomer: One of the units making up a polymer
Dehydration reaction: Monomers connected and covalently bonded by removing a hydrogen from both molecules and an oxygen from one (making H2O)

Hydrolysis: Bond between monomers of a polymer is broken by adding H2O

Carbohydrates
Contain elements: Carbon, hydrogen, and oxygen
Monomer: Monosaccharide
Polymer: Polysaccharides (chain of monosaccharides linked by covalent bonds called glycosidic linkages)
Functions: Provide and store energy, provide structural support
Important polysaccharides (all are glucose polymers)
Starch: Stores glucose in plants
Glycogen: Glucose storage in animals
Chitin: Structural polysaccharide in fungi cell wells and arthropod exoskeletons
Cellulose: Cell wall component for plant cells
Lipids
Elements: Carbon, hydrogen, oxygen (sometimes N or P)
No true monomers of lipids
Have little to no affinity for water (hydrophobic)
Function: Energy storage and structure
Saturated: No double bonds between carbon atoms, atoms tightly packed
Unsaturated: Double bonds between some carbons creating a kink in the chain

Trigylcerol: 3 fatty acids attached to one glycerol, energy storage
Phospholipid: Form phospholipid bilayer, have a hydrophilic head and hydrophobic tail
Cholesterol: Steroid that contributes to cell membrane integrity

Nucleic Acids
Elements: Carbon, hydrogen, oxygen, phosphorus
Monomers: Nucleotides, consist of a 5 carbon sugar, a phosphate, and a nitrogenous base

Polymers: Nucelic acids, linear sequences of nucleotides
Nitrogenous base pairs: Adenine pairs with thymine (or uracil in RNA), and cytosine pairs with guanine
Purines: Have a 2 ring structure (A and G)
Pyrmidines: Have a 1 ring structure ( C and T/U)

C and G are joined by 3 bonds, A and T are joined by 2
Directionality: Determined by orientation of sugars (hydroxyl = 3´ and phosphate = 5´)
Base pairs are joined by hydrogen bonds, nucleotides joined by covalent bonds

DNA: Antiparallel double helix (2 strands running in opposite directions), has deoxyribose
RNA: Single stranded, has ribose
Proteins
Elements: Carbon, hydrogen, oxygen, nitrogen, sulfur
Monomers: Amino acids, consist of carboxyl group + amino group + R group
Different amino acids have different R groups

Polymer: Polypeptide
STRUCTURE
Primary: Linear sequence of covalently bonded amino acids
Secondary: Structure formed by interactions between polypeptide´s ¨Backbone¨ ( carboxyl and amino groups)
Tertiary: 3d structure formed by interactions ( hydrogen bonds, ionic bonds, disulfide bridge, hydrophobic, etc.) between R groups
Quaternary: More than one polypeptide chain joined together

 Knowt
Knowt
