
industrial manufacturing techniques
continues process
batch process
production of aspirin
ethyl ethanoate and aspirin are produced by a batch process in industry
in a chemical plant large quantities of reactants are brought together in a reactor during which time the product is produced
after a certain time the reactor is emptied and the product is separated from the reaction mixture and purified
the product is then dried and packaged
esters and their manufacture
these have the functional group -COO-
they have the name -yl- anote eg methyl methoate
both R groups form part of the same
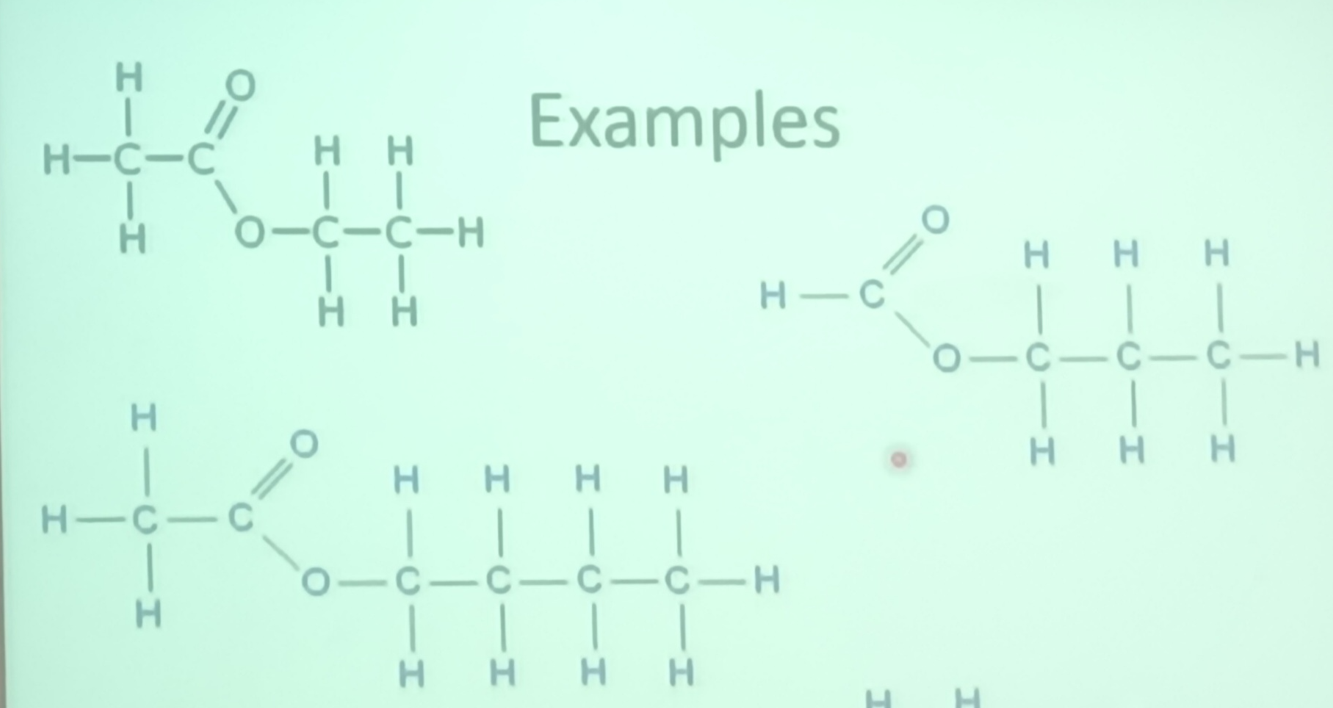
esters
esters are useful products
used as solvents
making polyesters
fruit smells are due to the presence of esters
ethyl ethanoate is used in glue and nail polish removers
ethyl ethanoate is used to remove caffeine from tea and coffee
manufacture
made by reacting ethanol with ethanoic acid
concentrated sulfuric acid is used as a catalyst
the reaction is slow and reversable
CH₃CH₂OH + CH₃COOH → CH₃COOCH₂CH₃ + H₂O
in industry the ester is made in a batch process
ethanol and ethanoic acid are added to a large reaction vessel together with the catalyst
the reaction mixture is heated
water produced during the rection is removed by distillation
yields of up to 95% are possible
other methods
Tishchenko reaction
liquid phase oxidation of butane
alkylation ethanoic acid with ethene (Avada process)
testing methods and techniques
boiling point determination
boiling point of pure substances are known very accurately and are listed in databases and books
the purity of a sample can be determined by comparing the boiling point of the sample to the actual value
boiling point occurs when the intermolecular bonds are broken
the greater the difference the more impurities are present
distillation can be used to determine boiling point
all values for boiling points are given as standard temperature and pressure
industrial manufacturing techniques
continues process
batch process
production of aspirin
ethyl ethanoate and aspirin are produced by a batch process in industry
in a chemical plant large quantities of reactants are brought together in a reactor during which time the product is produced
after a certain time the reactor is emptied and the product is separated from the reaction mixture and purified
the product is then dried and packaged
esters and their manufacture
these have the functional group -COO-
they have the name -yl- anote eg methyl methoate
both R groups form part of the same

esters
esters are useful products
used as solvents
making polyesters
fruit smells are due to the presence of esters
ethyl ethanoate is used in glue and nail polish removers
ethyl ethanoate is used to remove caffeine from tea and coffee
manufacture
made by reacting ethanol with ethanoic acid
concentrated sulfuric acid is used as a catalyst
the reaction is slow and reversable
CH₃CH₂OH + CH₃COOH → CH₃COOCH₂CH₃ + H₂O
in industry the ester is made in a batch process
ethanol and ethanoic acid are added to a large reaction vessel together with the catalyst
the reaction mixture is heated
water produced during the rection is removed by distillation
yields of up to 95% are possible
other methods
Tishchenko reaction
liquid phase oxidation of butane
alkylation ethanoic acid with ethene (Avada process)
testing methods and techniques
boiling point determination
boiling point of pure substances are known very accurately and are listed in databases and books
the purity of a sample can be determined by comparing the boiling point of the sample to the actual value
boiling point occurs when the intermolecular bonds are broken
the greater the difference the more impurities are present
distillation can be used to determine boiling point
all values for boiling points are given as standard temperature and pressure
 Knowt
Knowt
