
intermolecular forces (between)
the electrostatic interactions between molecules.
forces that exist between molecules.
(in strongest to weakest order)
HYDROGEN BONDING 🌊
H-FON
When hydrogen bonds with fluorine, oxygen, or nitrogen.
These are very strong dipole-dipole interactions as FON are the most electronegative elements, creating the most strongly polarized bonds.
The greater the difference in charge, the stronger the interaction (never stronger than formal charges ahem ions though.)
More hydrogen bonds can not occur unless the compound was already in a hydrogen bond originally or in no bond.
DIPOLE-DIPOLE INTERACTIONS 😡❤️😊
What is a dipole?
A bond or molecule whose ends have opposite charges.
Attractive forces between the positive end of one polar molecule and the negative end of another polar molecule.
Non-polar molecules/bonds cannot form dipole-dipole interactions as they have no charge.
Example of a dipole-dipole interaction
Water molecules are polar bc oxygen is more electronegative than hydrogen, thus, will pull the electrons in the bond towards itself. 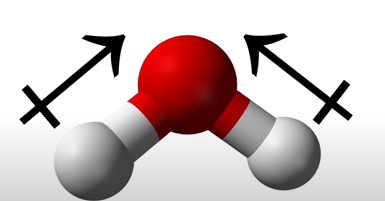
oxygen will have a negative charge as it has electron excess (needs to get rid of some)
hyrogens will have a positive charge as it has electron deficiency (lacking some)
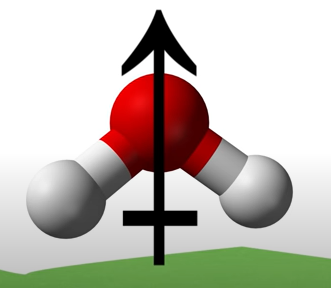
When the vectors (charges) are combined and one side has a stronger pull (electronegativity) than the other side, forming a dipole.
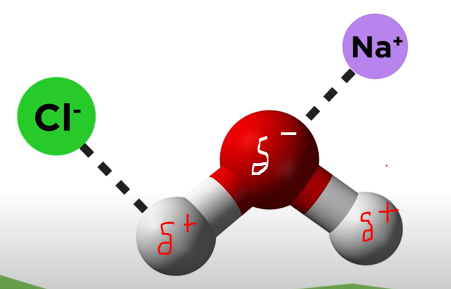
Ion-Dipole
When ions make interactions with atoms with the opposite charge.
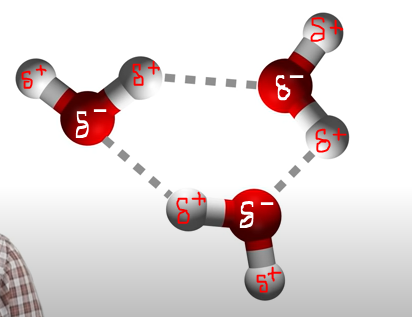
Dipole-dipole
molecules will move in such a way as to always be making electrostatic interactions between the negative end of one dipole and the positive end of another dipole.
DISPERSION FORCES 🁷🁷🁷🁷🁷🀷👈
DOMINOOOOOOS
it is what monoatomic species & non-polar covalent compounds can do.
a weak attraction occurs when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species.
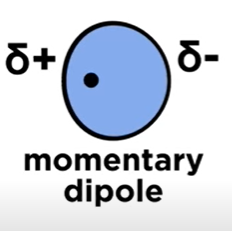
Momentary Dipole
When the electron cloud of an atom is SLIGHTLY lopsided, resulting in a small partial negative and positive charge.
Weaker than a formal dipole-dipole bond.
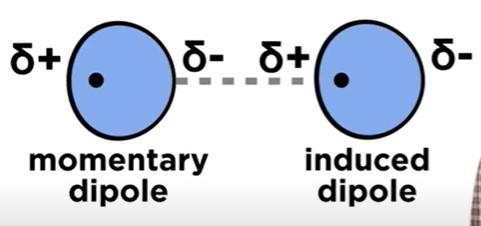
Induced Dipole
When momentary Dipole approaches another atom, the partial negativity gets repeled, moving to the other side of the atom and resulting in a slight dipole.
Thus, there will be a momentary dipole + induced dipole interaction.

intermolecular forces (between)
the electrostatic interactions between molecules.
forces that exist between molecules.
(in strongest to weakest order)
HYDROGEN BONDING 🌊
H-FON
When hydrogen bonds with fluorine, oxygen, or nitrogen.
These are very strong dipole-dipole interactions as FON are the most electronegative elements, creating the most strongly polarized bonds.
The greater the difference in charge, the stronger the interaction (never stronger than formal charges ahem ions though.)
More hydrogen bonds can not occur unless the compound was already in a hydrogen bond originally or in no bond.
DIPOLE-DIPOLE INTERACTIONS 😡❤️😊
What is a dipole?
A bond or molecule whose ends have opposite charges.
Attractive forces between the positive end of one polar molecule and the negative end of another polar molecule.
Non-polar molecules/bonds cannot form dipole-dipole interactions as they have no charge.
Example of a dipole-dipole interaction
Water molecules are polar bc oxygen is more electronegative than hydrogen, thus, will pull the electrons in the bond towards itself. 
oxygen will have a negative charge as it has electron excess (needs to get rid of some)
hyrogens will have a positive charge as it has electron deficiency (lacking some)
When the vectors (charges) are combined and one side has a stronger pull (electronegativity) than the other side, forming a dipole.

Ion-Dipole
When ions make interactions with atoms with the opposite charge.

Dipole-dipole
molecules will move in such a way as to always be making electrostatic interactions between the negative end of one dipole and the positive end of another dipole.

DISPERSION FORCES 🁷🁷🁷🁷🁷🀷👈
DOMINOOOOOOS
it is what monoatomic species & non-polar covalent compounds can do.
a weak attraction occurs when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species.
Momentary Dipole
When the electron cloud of an atom is SLIGHTLY lopsided, resulting in a small partial negative and positive charge.
Weaker than a formal dipole-dipole bond.

Induced Dipole
When momentary Dipole approaches another atom, the partial negativity gets repeled, moving to the other side of the atom and resulting in a slight dipole.
Thus, there will be a momentary dipole + induced dipole interaction.


 Knowt
Knowt