
Acids Bases and Salts
Acid and Base/Alkali Reactions
Acid + Metal → salt + hydrogen
Sulphuric acid + iron→ iron sulphate + hydrogen
Base/alkali + acid → salt + water
Sodium hydroxide + hydrochloric acid Sodium chloride + water
Acid + metal carbonate → salt + carbon dioxide + water
Nitric acid + magnesium oxide magnesium nitrate + carbon dioxide + water
Alkali + ammonium salt → salt + ammonia + water
Sodium hydroxide + ammonium chloride sodium chloride + ammonia + water
Acid: Proton Donors/ Dissociate into H+ ions
Have pH between 1 (strong) and 6 (weak)
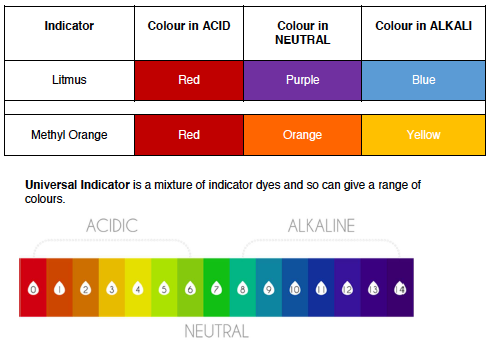
Turns blue litmus red
Turns methyl orange indicator red
Bases: Proton Acceptors/ Dissociate into OH- ions
Are the oxides and hydroxides of metals
Neutralise acids to give a salt and water only
Are mainly insoluble in water
Alkalis: (are bases that dissolve in water)
Feel soapy to the skin
Turn litmus blue
Gives solutions with a ph greater than 7
· Give solutions that contain OH – ions
Neutrality, acidity, alkalinity
pH scale runs from 1 – 14
Key facts:
Acids have a ph less than 7
The more acidic a solution, the lower the ph
Neutral substances, such as pure water have a ph of 7
Alkalis have ph greater than 7
Strong acid or base completely ionize in water
Weak acids partially ionize in water Ways to Measure pH:
Substances that change colour when added to an acid/alkali are called indicators. They are often in solution form but can also be found as paper
Metal and non-metal oxides
Acidic oxides: Non-metal oxides that dissolve in water and form an acidic solution
Basic oxides: Metal oxides that dissolve in water and form basic solution
Neutral oxides: Oxides with a pH of 7. Do not react with Acids or Alkalis
However there some exceptions for this rule, for example carbon monoxide.
Amphoteric oxides: those that react with both an acid and an alkali to give a salt and water.
eg. zinc and aluminum oxides
Preparing Salts
Preparing Soluble Salts
Method A: Neutralization
Excess insoluble compound (metal/base/carbonate) reacts with acid whilst being heated
Insoluble base is filtered out
Solution is heated in an evaporating dish to form soluble salt crystals
Method B: Titration
Phenolphthalein is added to an alkali (soluble base)
Add acid to solution using burette; note volume of acid required for solution to change color
Repeat without indictor using noted acid volume
Heat in evaporating dish to form soluble salt crystals
Preparing Insoluble Salts
Method C: Precipitation
2 soluble salts added to water and mixed
Note: one soluble salt should always be a potassium or sodium solution (eg. potassium sulfate)
Filter out and clean precipitate with distilled water
Dry insoluble salt precipitate in oven

Tests
Testing Cations
Cation | Aqueous NaOH | Aqueous Ammonia |
|---|---|---|
Aluminum (Al3+) | White soluble precipitate, turns colorless in excess | White precipitate, insoluble in excess |
Ammonium (NH4+) | Pungent ammonium gas produced turns damp red litmus blue | |
Calcium (Ca2+) | White precipitate, insoluble in excess | Faint or no precipitate |
Copper (Cu2+) | Blue precipitate, insoluble in excess | Blue precipitate, soluble in excess to give a dark blue solution |
Iron(II) (Fe2+) | green precipitate, insoluble in excess | green precipitate, soluble in excess |
Iron(III) (Fe3+) | Reddish-brown precipitate, insoluble in excess | Reddish-brown precipitate, insoluble in excess |
Zinc (Zn2+) | White precipitate, soluble and turns colorless in excess | White precipitate, soluble and turns colorless in excess |
Chromium (Cr3+) | Grey green precipitate, soluble to give dark green solution in excess | Grey green precipitate, insoluble in excess |
Testing for Anions:
Sulfate ions (SO42-):
Add dilute nitric acid, then add aq. barium nitrate
White precipitate formed
Sulphite ions (SO32-):
Add acidified potassium permanganate and heat
Color changes from pink to colorless
Halide ions:
Add nitric acid, then aqueous silver nitrate
Chloride (Cl-) | White precipitate |
|---|---|
Bromide (Br-) | Cream precipitate |
Iodide (I-) | Yellow precipitate |
Nitrate ions (NO3-):
Add aqueous sodium hydroxide then add warm aluminum foil
Pungent gas produced, turns damp red litmus blue
Carbonate ions (CO32-):
Add dilute hydrochloric acid
If bubbles/ gas produced turn limewater cloudy, carbonate ion present
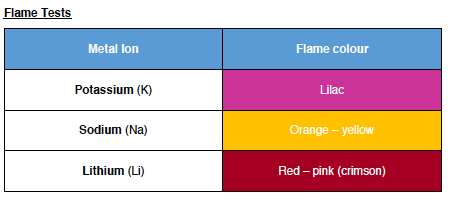
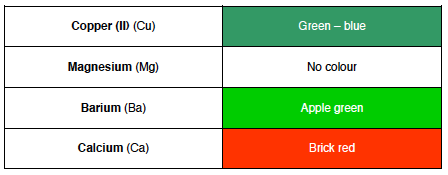
Flame Tests
Test for Gases:
Gas | Test result |
|---|---|
Ammonia (NH3) | Damp red litmus paper turns blue |
Carbon dioxide (CO2) | Bubble gas through–from colorless to cloudy |
Chlorine (Cl2) | Bleaches red/blue litmus paper |
Hydrogen (H2) | Place lighted splint, squeaky pop |
Oxygen (O2) | Place glowing splint, splint relights |
Acids Bases and Salts
Acid and Base/Alkali Reactions
Acid + Metal → salt + hydrogen
Sulphuric acid + iron→ iron sulphate + hydrogen
Base/alkali + acid → salt + water
Sodium hydroxide + hydrochloric acid Sodium chloride + water
Acid + metal carbonate → salt + carbon dioxide + water
Nitric acid + magnesium oxide magnesium nitrate + carbon dioxide + water
Alkali + ammonium salt → salt + ammonia + water
Sodium hydroxide + ammonium chloride sodium chloride + ammonia + water
Acid: Proton Donors/ Dissociate into H+ ions
Have pH between 1 (strong) and 6 (weak)
Turns blue litmus red
Turns methyl orange indicator red
Bases: Proton Acceptors/ Dissociate into OH- ions
Are the oxides and hydroxides of metals
Neutralise acids to give a salt and water only
Are mainly insoluble in water
Alkalis: (are bases that dissolve in water)
Feel soapy to the skin
Turn litmus blue
Gives solutions with a ph greater than 7
· Give solutions that contain OH – ions
Neutrality, acidity, alkalinity
pH scale runs from 1 – 14
Key facts:
Acids have a ph less than 7
The more acidic a solution, the lower the ph
Neutral substances, such as pure water have a ph of 7
Alkalis have ph greater than 7
Strong acid or base completely ionize in water
Weak acids partially ionize in water Ways to Measure pH:
Substances that change colour when added to an acid/alkali are called indicators. They are often in solution form but can also be found as paper

Metal and non-metal oxides
Acidic oxides: Non-metal oxides that dissolve in water and form an acidic solution
Basic oxides: Metal oxides that dissolve in water and form basic solution
Neutral oxides: Oxides with a pH of 7. Do not react with Acids or Alkalis
However there some exceptions for this rule, for example carbon monoxide.
Amphoteric oxides: those that react with both an acid and an alkali to give a salt and water.
eg. zinc and aluminum oxides
Preparing Salts
Preparing Soluble Salts
Method A: Neutralization
Excess insoluble compound (metal/base/carbonate) reacts with acid whilst being heated
Insoluble base is filtered out
Solution is heated in an evaporating dish to form soluble salt crystals
Method B: Titration
Phenolphthalein is added to an alkali (soluble base)
Add acid to solution using burette; note volume of acid required for solution to change color
Repeat without indictor using noted acid volume
Heat in evaporating dish to form soluble salt crystals
Preparing Insoluble Salts
Method C: Precipitation
2 soluble salts added to water and mixed
Note: one soluble salt should always be a potassium or sodium solution (eg. potassium sulfate)
Filter out and clean precipitate with distilled water
Dry insoluble salt precipitate in oven

Tests
Testing Cations
Cation | Aqueous NaOH | Aqueous Ammonia |
|---|---|---|
Aluminum (Al3+) | White soluble precipitate, turns colorless in excess | White precipitate, insoluble in excess |
Ammonium (NH4+) | Pungent ammonium gas produced turns damp red litmus blue | |
Calcium (Ca2+) | White precipitate, insoluble in excess | Faint or no precipitate |
Copper (Cu2+) | Blue precipitate, insoluble in excess | Blue precipitate, soluble in excess to give a dark blue solution |
Iron(II) (Fe2+) | green precipitate, insoluble in excess | green precipitate, soluble in excess |
Iron(III) (Fe3+) | Reddish-brown precipitate, insoluble in excess | Reddish-brown precipitate, insoluble in excess |
Zinc (Zn2+) | White precipitate, soluble and turns colorless in excess | White precipitate, soluble and turns colorless in excess |
Chromium (Cr3+) | Grey green precipitate, soluble to give dark green solution in excess | Grey green precipitate, insoluble in excess |
Testing for Anions:
Sulfate ions (SO42-):
Add dilute nitric acid, then add aq. barium nitrate
White precipitate formed
Sulphite ions (SO32-):
Add acidified potassium permanganate and heat
Color changes from pink to colorless
Halide ions:
Add nitric acid, then aqueous silver nitrate
Chloride (Cl-) | White precipitate |
|---|---|
Bromide (Br-) | Cream precipitate |
Iodide (I-) | Yellow precipitate |
Nitrate ions (NO3-):
Add aqueous sodium hydroxide then add warm aluminum foil
Pungent gas produced, turns damp red litmus blue
Carbonate ions (CO32-):
Add dilute hydrochloric acid
If bubbles/ gas produced turn limewater cloudy, carbonate ion present
Flame Tests


Test for Gases:
Gas | Test result |
|---|---|
Ammonia (NH3) | Damp red litmus paper turns blue |
Carbon dioxide (CO2) | Bubble gas through–from colorless to cloudy |
Chlorine (Cl2) | Bleaches red/blue litmus paper |
Hydrogen (H2) | Place lighted splint, squeaky pop |
Oxygen (O2) | Place glowing splint, splint relights |
 Knowt
Knowt
