
1.4 Alcohols
Alcohols
General Formula: R-OH
Name of the functional group: Hydroxyl
Naming Alcohols
Add “ol” to the end of the alkane name.
Alcohol Types
Primary (1°) alcohols – hydroxyl is bonded to a terminal carbon
Secondary (2°) alcohols – hydroxyl is bonded to a carbon attached to 2 alkyl groups (Cs)
Tertiary (3°) alcohols – hydroxyl is bonded to a carbon attached to 3 alkyl groups (Cs)

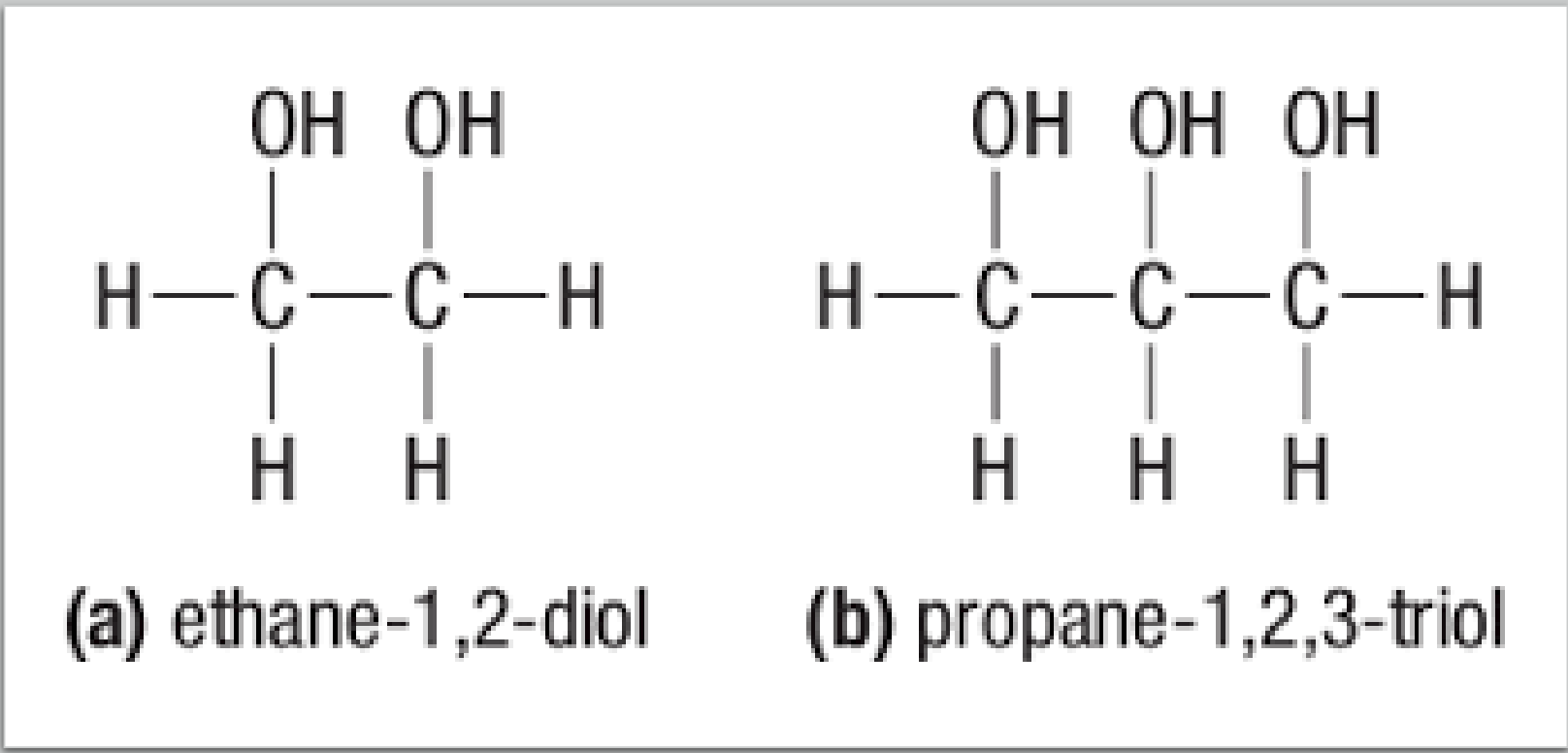
Polyalcohols: alcohols with more than one hydroxyl group
Cyclic alcohols: cyclohexanol
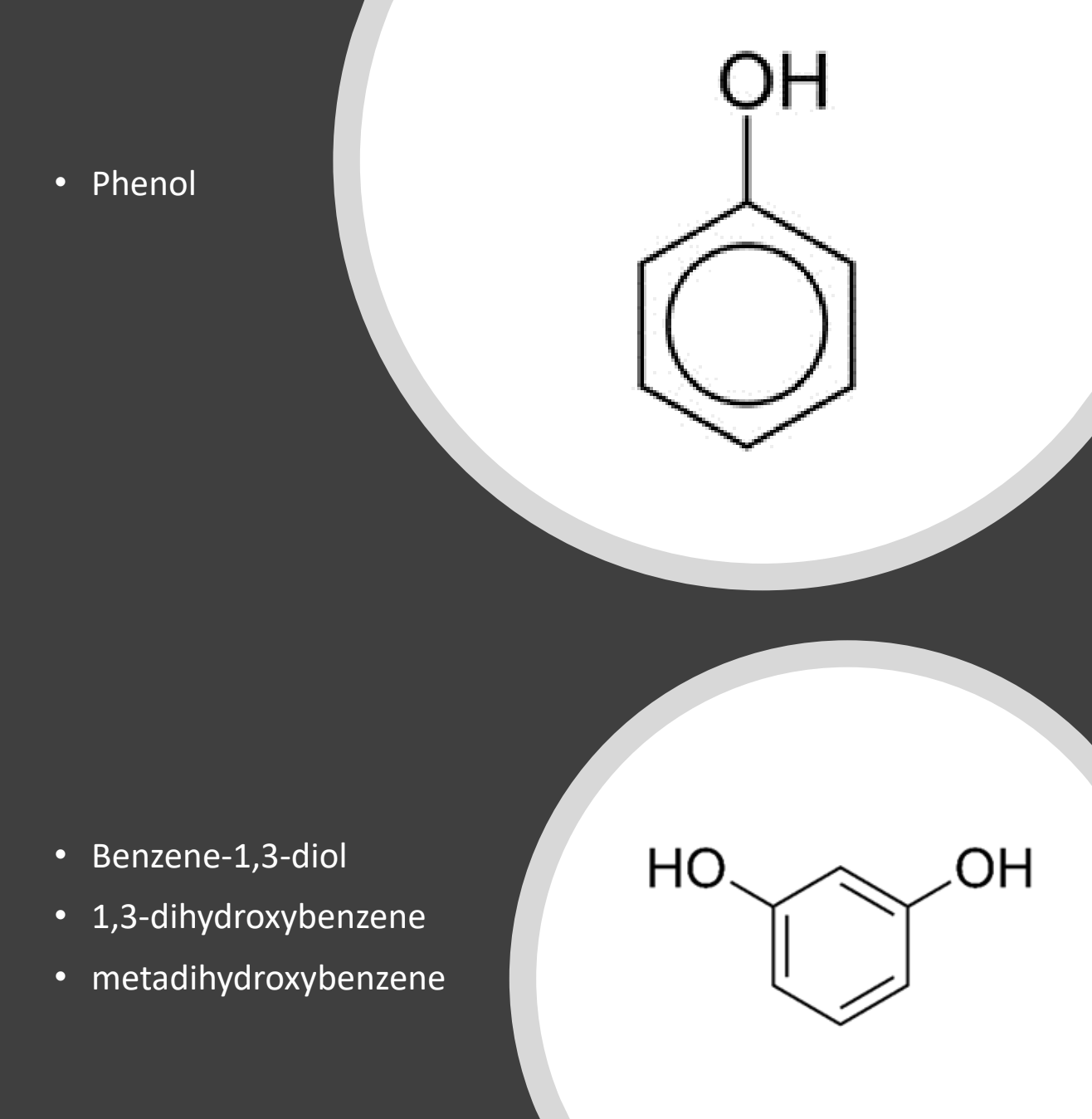
Aromatic alcohols:
Properties of Alcohols
Alcohols have a much higher boiling point than alkanes because of the hydroxyl group
This makes the alcohol molecule polar and allows them to form hydrogen bonds, increasing the intermolecular forces
Small alcohols have a high solubility in polar solvents (water)
In long-chain alcohols, the hydrocarbon portion is nonpolar making larger alcohols good solvents for non-polar compounds.
Reactions
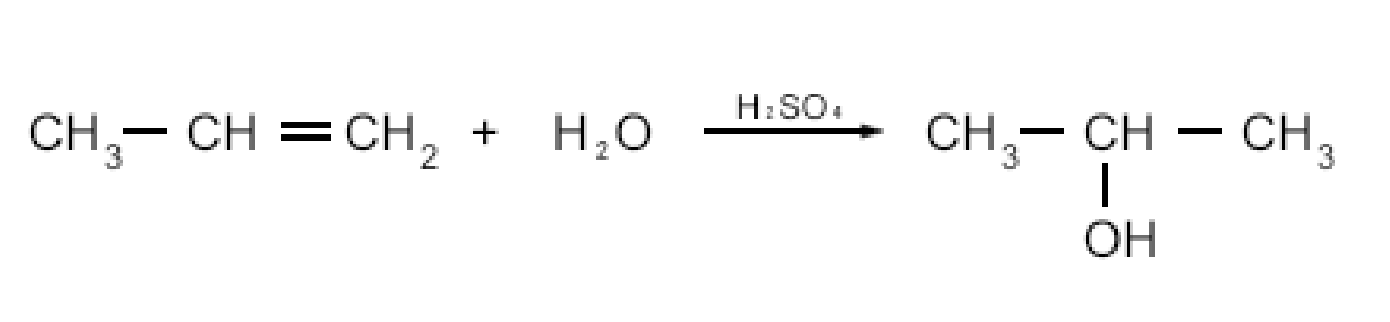
Hydration of alkenes
Requires a catalyst such as H2SO4
Addition reaction
Makes 2 & 3 alcohols (Markovnikov’s rule)
Hydration of alkyl halides
Substitution reaction
Makes 1 alcohols

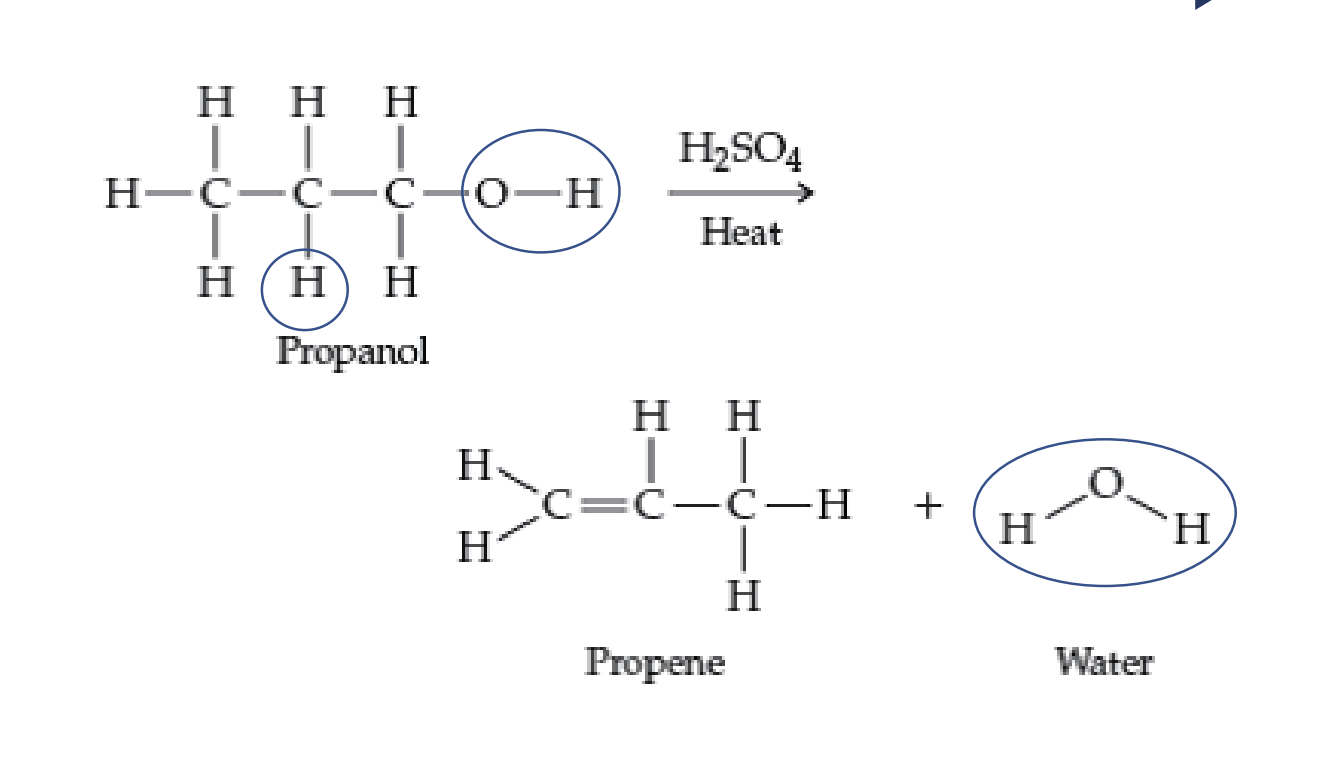
Elimination/Dehydration
Prepares alkenes from alcohols
Requires catalyst (H2SO4)
Combustion of Alcohols
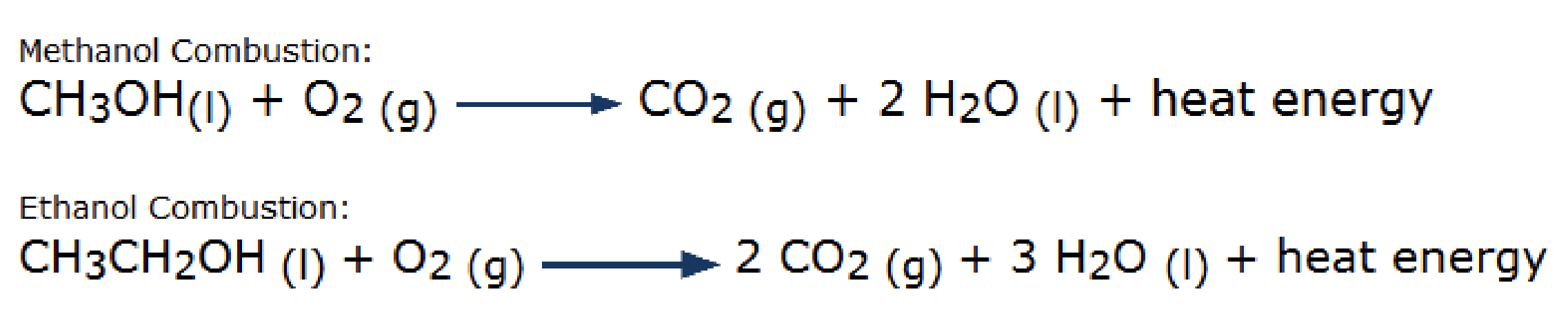
1.4 Alcohols
Alcohols
General Formula: R-OH
Name of the functional group: Hydroxyl
Naming Alcohols
Add “ol” to the end of the alkane name.
Alcohol Types
Primary (1°) alcohols – hydroxyl is bonded to a terminal carbon
Secondary (2°) alcohols – hydroxyl is bonded to a carbon attached to 2 alkyl groups (Cs)
Tertiary (3°) alcohols – hydroxyl is bonded to a carbon attached to 3 alkyl groups (Cs)

Polyalcohols: alcohols with more than one hydroxyl group

Cyclic alcohols: cyclohexanol
Aromatic alcohols:

Properties of Alcohols
Alcohols have a much higher boiling point than alkanes because of the hydroxyl group
This makes the alcohol molecule polar and allows them to form hydrogen bonds, increasing the intermolecular forces
Small alcohols have a high solubility in polar solvents (water)
In long-chain alcohols, the hydrocarbon portion is nonpolar making larger alcohols good solvents for non-polar compounds.
Reactions
Hydration of alkenes
Requires a catalyst such as H2SO4
Addition reaction
Makes 2 & 3 alcohols (Markovnikov’s rule)

Hydration of alkyl halides
Substitution reaction
Makes 1 alcohols

Elimination/Dehydration
Prepares alkenes from alcohols
Requires catalyst (H2SO4)

Combustion of Alcohols
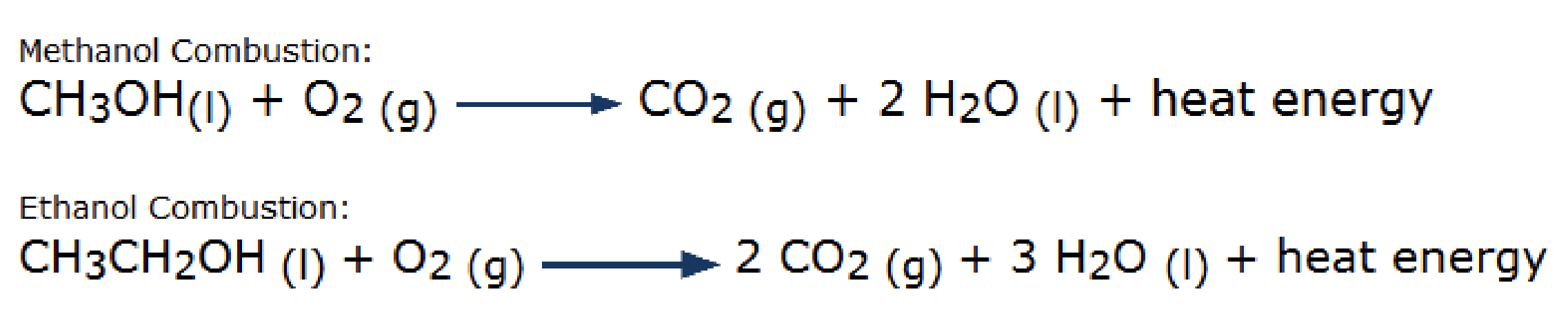
 Knowt
Knowt
