Periodic Table and Trends
Johann Dobereiner (1780-1849)
In 1829, classified some elements into groups of three (He called them triads)
The elements in the triads had similar chemical properties & orderly physical properties
Model of Triads
John Newlands (1839-1898)
In 1863, he suggested that elements be arranged in “octaves”
He noticed (after arranging the elements in order of increasing atomic mass) that certain properties repeated every 8th element
Law of Octaves
Claimed to see a repeating pattern was met with savage ridicule on its announcement
His classification of the elements was as arbitrary as putting them in alphabetical order and his paper was rejected for publication by the Chemical Society
Dmitri Mendeleev
A Russian Chemist and Inventor
Published the periodic table in the form we use today
His periodic table grouped similar elements into columns (Just like ours does today)
Lothar Meyer (1830-1895)
At the same time as Mendeleev, he published his own table of elements
He organized the elements by increasing atomic mass
Periodic Table
Elements on the table can be divided into three main categories: Metals, Non-Metals, and Metalloids
The periodic Repetition of chemical properties is the result of the arrangement of electrons in the outer energy level (Valence Electrons)
Variations in physical properties are due to different atomic numbers (Protons)
Elements on the periodic table can be grouped into families based on their chemical properties
Each family has a specific name to differentiate it from the other families in the periodic table
Elements in each family react differently with other elements
The horizontals rows are called periods and are labeled 1 to 7
the vertical columns are called groups/families and are labeled 1 to 18
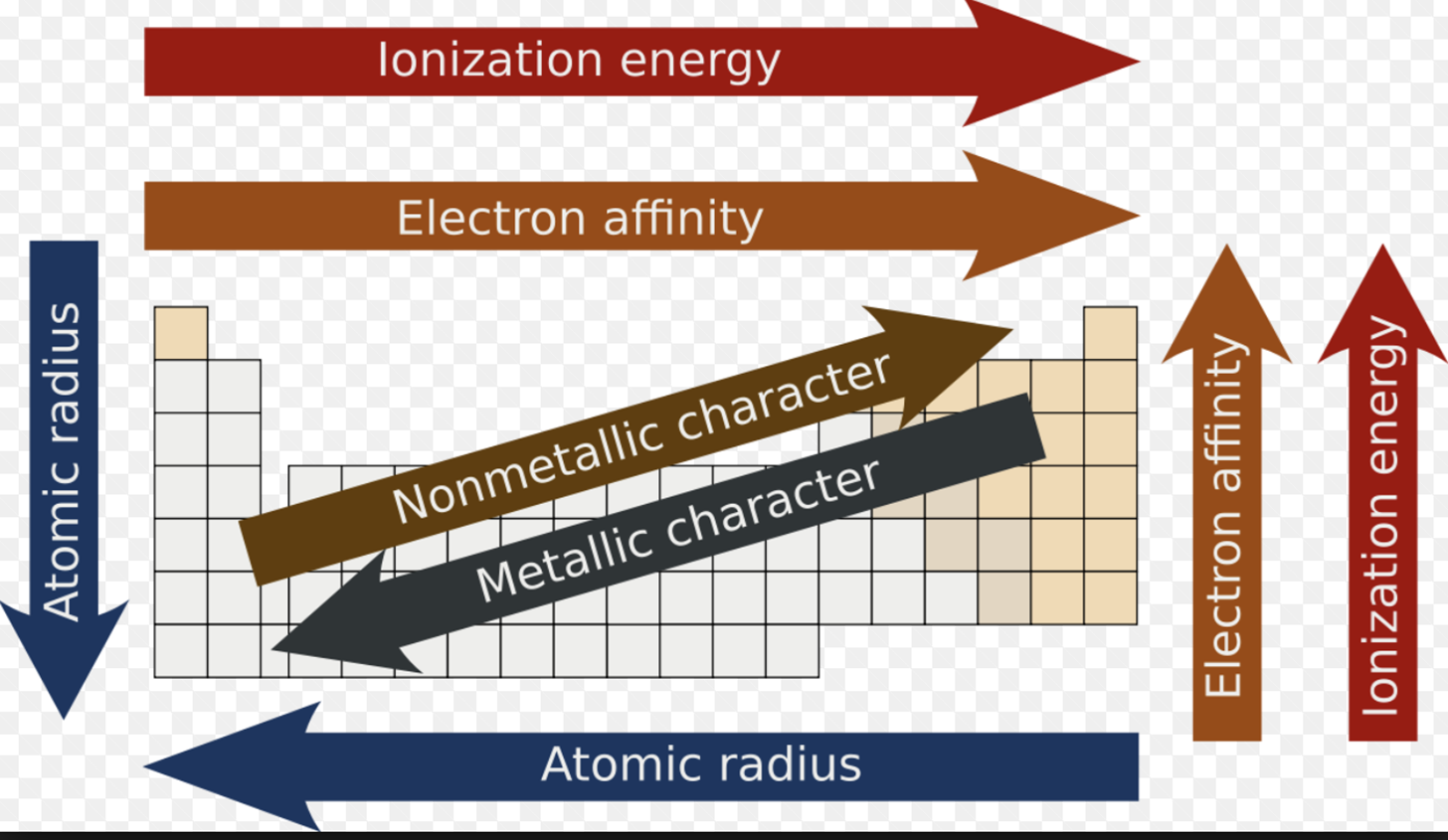
Metals
Solid at room temperature (Except for Mercury - it is a liquid)
Shiny lustre
Good conductors of heat and electricity
Malleable
Ductile
Non-Metals
They are a gas or a solid at room temperature (Bromine is the only one that is a liquid)
Not very shiny
Poor conductors of heat and electricity
Brittle
Not ductile
Metalloids
Solid at room temperature
Can be shiny or dull
May conduct electricity
Poor conductors of heat
Brittle
Not ductile
Families
Hydrogen - Belongs to family of its own; a diatomic reactive gas; involved in the explosion of the Hindenburg; promising as an alternative fuel source for automobile
Alkali Metals - Hydrogen is not a member, it is a non-metal; 1 electron in the outer shell (One valence electron); soft silvery metals; Very reactive, especially with water; conduct electricity
Alkaline Earth Metals - 2 electrons in the outer shell (Two valence electrons); white and malleable; reactive, but less than Alkali metals; conduct electricity
Transition Metals - Good conductors of heat and electricity; some are used for jewelry; the transition metals are able to put up to 32 electrons in their second to last shell; can bond with many elements in a variety of shapes
Boron Family - 3 electrons in the outer shell (three valence electrons); most are metals; Boron is a metalloid
Carbon Family - 4 electrons in the outer shell (four valence electrons); Contains metals, metalloids, and a non-metal (carbon) (C)
Nitrogen Family - 5 electrons in the outer shell (five valence electrons); can share electrons to form compounds; contains metals, metalloids, and non-metals
Oxygen Family (Chalcogens) - 6 electrons in the outer shell (six valence electrons); contains, metals, metalloids, and non-metals; reactive
Halogens - 7 electrons in the outer shell (seven valence electrons); all are non-metals; Very reactive are often bonded with the elements from Group 1
Noble Gases - Exist as gases; non-metals; 8 electrons in the outer shell = Full; Helium (He) has only 2 electrons in the outer shell = Full; Not reactive with other elements
Rare Earth Metals (Lanthanide & Actinide) - Some are radioactive; silver, silvery-white, or grey metals; conduct electricity
Ions
When an atom loses or gains electrons
Cations are positive and are formed by elements on the left side of the periodic chart
Anions are negative and are formed by elements on the right side of the periodic chart
Zeff
Typically refer to the effective nuclear charge
Increase to the right
Increases going up
Atomic Radii
Half the distance between the nuclei of identical atoms that are bonded together
Defined by the edge of its orbital but since the edges are fuzzy, it is difficult to determine
Atomic Size
Increase size going down
Decrease size going to the right
Electrons are in the same energy level
But, there is more nuclear charge
Outermost electrons are pulled closer
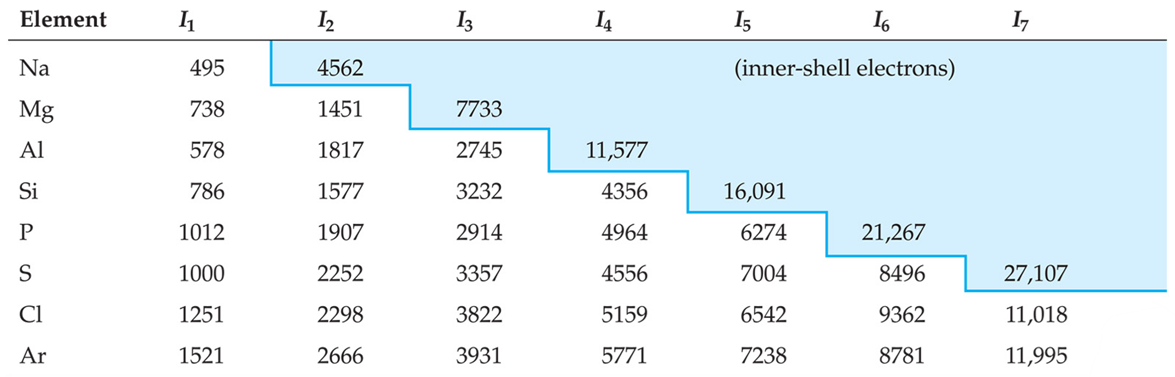
Ionization Energy (Ei)
Minimum energy required to remove an electron from the ground state of atom (molecule) in the gas phase
Frist Ionization Energy - the energy needed to remove the outermost electron from an atom
Second Ionization Energy - the energy needed to remove the second electron from an atom, etc.
The greater the nuclear charge, the greater IE
Greater distance from nucleus decreases IE
Increases moving to the right
Increases going up (If it moves down the electron is further away from the nucleus, having less pull on it)
Electronegativity
Measure of an attraction of an atom for a shared electron
Electronegativity is the tendency for an atom to attract electrons to itself when it is chemically combined with another element
An element with a big electronegativity means it pulls the electron towards itself strongly!
This is the small table on the back of your periodic table
Ionic Radius
The element’s share of the distance between neighboring ions in an ionic solid
Generally: Cations are smaller than their parent atoms and Anions are larger than their parent atoms
Electron Affinity
The energy change associated with the addition of an electron
Increases going the right
Increase going up
Periodic Table and Trends
Johann Dobereiner (1780-1849)
In 1829, classified some elements into groups of three (He called them triads)
The elements in the triads had similar chemical properties & orderly physical properties
Model of Triads
John Newlands (1839-1898)
In 1863, he suggested that elements be arranged in “octaves”
He noticed (after arranging the elements in order of increasing atomic mass) that certain properties repeated every 8th element
Law of Octaves
Claimed to see a repeating pattern was met with savage ridicule on its announcement
His classification of the elements was as arbitrary as putting them in alphabetical order and his paper was rejected for publication by the Chemical Society
Dmitri Mendeleev
A Russian Chemist and Inventor
Published the periodic table in the form we use today
His periodic table grouped similar elements into columns (Just like ours does today)
Lothar Meyer (1830-1895)
At the same time as Mendeleev, he published his own table of elements
He organized the elements by increasing atomic mass
Periodic Table
Elements on the table can be divided into three main categories: Metals, Non-Metals, and Metalloids
The periodic Repetition of chemical properties is the result of the arrangement of electrons in the outer energy level (Valence Electrons)
Variations in physical properties are due to different atomic numbers (Protons)
Elements on the periodic table can be grouped into families based on their chemical properties
Each family has a specific name to differentiate it from the other families in the periodic table
Elements in each family react differently with other elements
The horizontals rows are called periods and are labeled 1 to 7
the vertical columns are called groups/families and are labeled 1 to 18
Metals
Solid at room temperature (Except for Mercury - it is a liquid)
Shiny lustre
Good conductors of heat and electricity
Malleable
Ductile
Non-Metals
They are a gas or a solid at room temperature (Bromine is the only one that is a liquid)
Not very shiny
Poor conductors of heat and electricity
Brittle
Not ductile
Metalloids
Solid at room temperature
Can be shiny or dull
May conduct electricity
Poor conductors of heat
Brittle
Not ductile
Families
Hydrogen - Belongs to family of its own; a diatomic reactive gas; involved in the explosion of the Hindenburg; promising as an alternative fuel source for automobile
Alkali Metals - Hydrogen is not a member, it is a non-metal; 1 electron in the outer shell (One valence electron); soft silvery metals; Very reactive, especially with water; conduct electricity
Alkaline Earth Metals - 2 electrons in the outer shell (Two valence electrons); white and malleable; reactive, but less than Alkali metals; conduct electricity
Transition Metals - Good conductors of heat and electricity; some are used for jewelry; the transition metals are able to put up to 32 electrons in their second to last shell; can bond with many elements in a variety of shapes
Boron Family - 3 electrons in the outer shell (three valence electrons); most are metals; Boron is a metalloid
Carbon Family - 4 electrons in the outer shell (four valence electrons); Contains metals, metalloids, and a non-metal (carbon) (C)
Nitrogen Family - 5 electrons in the outer shell (five valence electrons); can share electrons to form compounds; contains metals, metalloids, and non-metals
Oxygen Family (Chalcogens) - 6 electrons in the outer shell (six valence electrons); contains, metals, metalloids, and non-metals; reactive
Halogens - 7 electrons in the outer shell (seven valence electrons); all are non-metals; Very reactive are often bonded with the elements from Group 1
Noble Gases - Exist as gases; non-metals; 8 electrons in the outer shell = Full; Helium (He) has only 2 electrons in the outer shell = Full; Not reactive with other elements
Rare Earth Metals (Lanthanide & Actinide) - Some are radioactive; silver, silvery-white, or grey metals; conduct electricity
Ions
When an atom loses or gains electrons
Cations are positive and are formed by elements on the left side of the periodic chart
Anions are negative and are formed by elements on the right side of the periodic chart
Zeff
Typically refer to the effective nuclear charge
Increase to the right
Increases going up
Atomic Radii
Half the distance between the nuclei of identical atoms that are bonded together
Defined by the edge of its orbital but since the edges are fuzzy, it is difficult to determine
Atomic Size
Increase size going down
Decrease size going to the right
Electrons are in the same energy level
But, there is more nuclear charge
Outermost electrons are pulled closer
Ionization Energy (Ei)
Minimum energy required to remove an electron from the ground state of atom (molecule) in the gas phase
Frist Ionization Energy - the energy needed to remove the outermost electron from an atom
Second Ionization Energy - the energy needed to remove the second electron from an atom, etc.
The greater the nuclear charge, the greater IE
Greater distance from nucleus decreases IE
Increases moving to the right
Increases going up (If it moves down the electron is further away from the nucleus, having less pull on it)

Electronegativity
Measure of an attraction of an atom for a shared electron
Electronegativity is the tendency for an atom to attract electrons to itself when it is chemically combined with another element
An element with a big electronegativity means it pulls the electron towards itself strongly!
This is the small table on the back of your periodic table
Ionic Radius
The element’s share of the distance between neighboring ions in an ionic solid
Generally: Cations are smaller than their parent atoms and Anions are larger than their parent atoms
Electron Affinity
The energy change associated with the addition of an electron
Increases going the right
Increase going up
 Knowt
Knowt