
AP Chem: Chapter 7 - Thermochemistry
Important Vocab
Thermochemistry: the study of the relationships between chemistry and energy
Energy: the capacity to do work
Work: the result of a force acting through a distance
Thermodynamics: study of energy and its interconversions
Internal energy: the sum of the kinetic and potential energies of all of the particles that compose the system
Pressure–volume work: occurs when a force (caused by a change in volume) acts through a distance against an external pressure
Heat: the transfer of thermal energy
Thermal equilibrium: Surroundings & object are same temp, no additional net transfer of temp
Heat capacity: the quantity of heat required to change its temperature by 1 °C
Calorimetry: measure the thermal energy exchanged between the reaction (defined as the system) and the surroundings by observing the change in temperature of the surroundings
Enthalpy: the sum of a system’s internal energy and the product of its pressure and volume
Types of Energy
Kinetic
Associated with the motion of an object
Ex: Moving ball
Thermal
Associated with the temperature of an object
Type of kinetic energy
Arises from the motions of atoms or molecules within a substance
Ex: Hot cup of coffee
Potential
Associated with the position or composition of an object
Ex: compressed spring, ball held up above the ground
Chemical
Type of potential energy
Often stored in chemical bonds
Associated with the relative positions of electrons and nuclei in atoms and molecules
Thermodynamics
First Law of Thermodynamics
Also known as the law of energy conservation
Energy is neither created nor destroyed
Internal energy: the sum of the kinetic and potential energies of all of the particles that compose the system
Internal energy is a state system (value depends only on the state of the system)
Energy flow rules:
Reactants have a higher internal energy than the products, is negative and energy flows out of the system into the surroundings
If the reactants have a lower internal energy than the products, is positive and energy flows into the system from the surroundings
Heat
Heat: the transfer of thermal energy
Thermal equilibrium: Surroundings & object are same temp, no additional net transfer of temp
Heat capacity: the quantity of heat required to change its temperature by 1 °C
Depends on:
The amount of matter being heated
Specific heat capacity/molar capacity (q)
Pressure–volume work: occurs when a force (caused by a change in volume) acts through a distance against an external pressure
w = F * D
Calorimetry: measure the thermal energy exchanged between the reaction (defined as the system) and the surroundings by observing the change in temperature of the surroundings
Measurement tool: bomb calorimeter and coffee-cup calorimeter
Bomb calorimetry occurs at constant volume and measures ΔE for a reaction
Coffee-cup calorimetry occurs at constant pressure and measures ΔH for a reaction
Enthalpy
Enthalpy: the sum of a system’s internal energy and the product of its pressure and volume
H = E + PV
Negative delta H = endothermic reaction
Positive delta H = exothermic reaction
The value of ΔH for a chemical reaction is the amount of heat absorbed or evolved in the reaction under conditions of constant pressure
An endothermic reaction has a positive ΔH and absorbs heat from the surroundings. An endothermic reaction feels cold to the touch
An exothermic reaction has a negative ΔH and gives off heat to the surroundings. An exothermic reaction feels warm to the touch
Standard heat of formation
Standard State
For a Gas: The standard state for a gas is the pure gas at a pressure of exactly 1 atm.
For a Liquid or Solid: The standard state for a liquid or solid is the pure substance in its most stable form at a pressure of 1 atm and at the temperature of interest (often taken to be 25 °C).
For a Substance in Solution: The standard state for a substance in solution is a concentration of exactly 1 M.
Standard Enthalpy Change (ΔH°)
The change in enthalpy for a process when all reactants and products are in their standard states. The degree sign indicates standard states.
Standard Enthalpy of Formation ()
For a Pure Compound: The change in enthalpy when 1 mol of the compound forms from its constituent elements in their standard states.
For a Pure Element in Its Standard State: delta H = 0
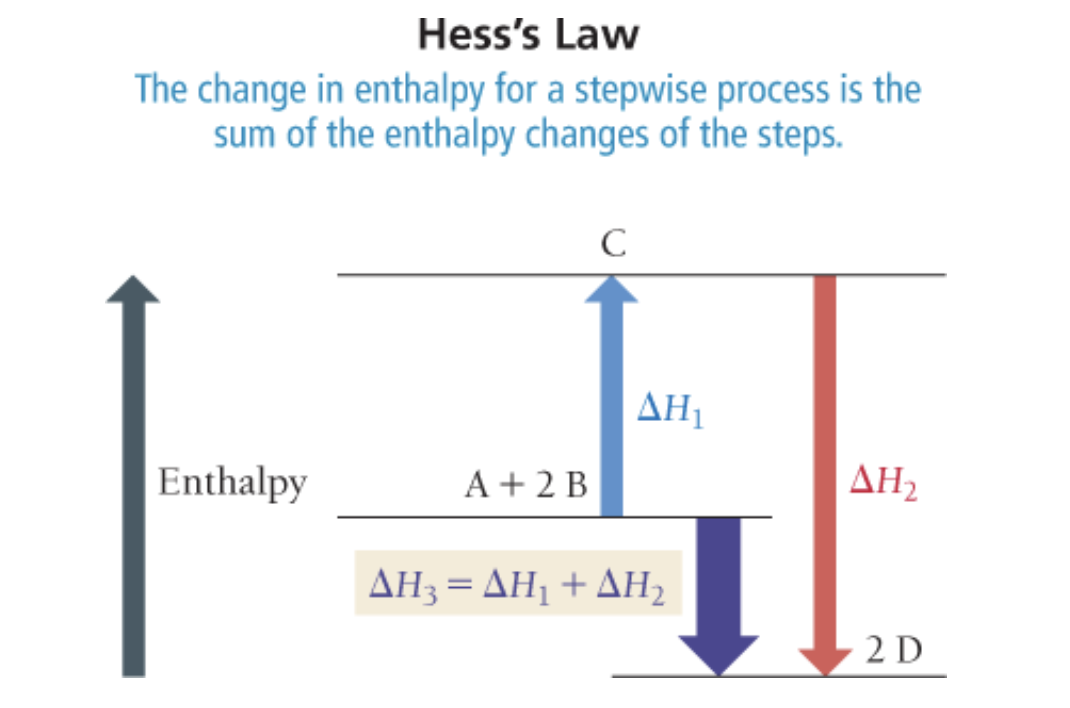
Hess’ Law
If a chemical equation can be expressed as the sum of a series of steps, then for the overall equation is the sum of the heats of reaction for each step
AP Chem: Chapter 7 - Thermochemistry
Important Vocab
Thermochemistry: the study of the relationships between chemistry and energy
Energy: the capacity to do work
Work: the result of a force acting through a distance
Thermodynamics: study of energy and its interconversions
Internal energy: the sum of the kinetic and potential energies of all of the particles that compose the system
Pressure–volume work: occurs when a force (caused by a change in volume) acts through a distance against an external pressure
Heat: the transfer of thermal energy
Thermal equilibrium: Surroundings & object are same temp, no additional net transfer of temp
Heat capacity: the quantity of heat required to change its temperature by 1 °C
Calorimetry: measure the thermal energy exchanged between the reaction (defined as the system) and the surroundings by observing the change in temperature of the surroundings
Enthalpy: the sum of a system’s internal energy and the product of its pressure and volume
Types of Energy
Kinetic
Associated with the motion of an object
Ex: Moving ball
Thermal
Associated with the temperature of an object
Type of kinetic energy
Arises from the motions of atoms or molecules within a substance
Ex: Hot cup of coffee
Potential
Associated with the position or composition of an object
Ex: compressed spring, ball held up above the ground
Chemical
Type of potential energy
Often stored in chemical bonds
Associated with the relative positions of electrons and nuclei in atoms and molecules
Thermodynamics
First Law of Thermodynamics
Also known as the law of energy conservation
Energy is neither created nor destroyed
Internal energy: the sum of the kinetic and potential energies of all of the particles that compose the system
Internal energy is a state system (value depends only on the state of the system)
Energy flow rules:
Reactants have a higher internal energy than the products, is negative and energy flows out of the system into the surroundings
If the reactants have a lower internal energy than the products, is positive and energy flows into the system from the surroundings
Heat
Heat: the transfer of thermal energy
Thermal equilibrium: Surroundings & object are same temp, no additional net transfer of temp
Heat capacity: the quantity of heat required to change its temperature by 1 °C
Depends on:
The amount of matter being heated
Specific heat capacity/molar capacity (q)
Pressure–volume work: occurs when a force (caused by a change in volume) acts through a distance against an external pressure
w = F * D
Calorimetry: measure the thermal energy exchanged between the reaction (defined as the system) and the surroundings by observing the change in temperature of the surroundings
Measurement tool: bomb calorimeter and coffee-cup calorimeter
Bomb calorimetry occurs at constant volume and measures ΔE for a reaction
Coffee-cup calorimetry occurs at constant pressure and measures ΔH for a reaction
Enthalpy
Enthalpy: the sum of a system’s internal energy and the product of its pressure and volume
H = E + PV
Negative delta H = endothermic reaction
Positive delta H = exothermic reaction
The value of ΔH for a chemical reaction is the amount of heat absorbed or evolved in the reaction under conditions of constant pressure
An endothermic reaction has a positive ΔH and absorbs heat from the surroundings. An endothermic reaction feels cold to the touch
An exothermic reaction has a negative ΔH and gives off heat to the surroundings. An exothermic reaction feels warm to the touch
Standard heat of formation
Standard State
For a Gas: The standard state for a gas is the pure gas at a pressure of exactly 1 atm.
For a Liquid or Solid: The standard state for a liquid or solid is the pure substance in its most stable form at a pressure of 1 atm and at the temperature of interest (often taken to be 25 °C).
For a Substance in Solution: The standard state for a substance in solution is a concentration of exactly 1 M.
Standard Enthalpy Change (ΔH°)
The change in enthalpy for a process when all reactants and products are in their standard states. The degree sign indicates standard states.
Standard Enthalpy of Formation ()
For a Pure Compound: The change in enthalpy when 1 mol of the compound forms from its constituent elements in their standard states.
For a Pure Element in Its Standard State: delta H = 0
Hess’ Law
If a chemical equation can be expressed as the sum of a series of steps, then for the overall equation is the sum of the heats of reaction for each step

 Knowt
Knowt