
Atomic Structure
Model of the Atom
Dalton’s Model (1803)- Suggested that all matter is made of atoms and atoms cannot be split.
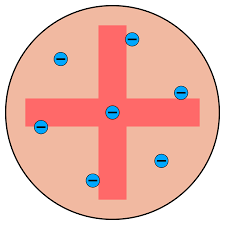
Thomson’s Plum Pudding Model (1897)- Discovered electrons. He created the ‘plum pudding’ model, a ball of positive charge with negatively charged electrons.
Rutherford’s nuclear Model(1909)- discovered that alpha particles can bounce back off atoms. Concluded that the atoms mass is concentrated.
Niels Bohr(1932)- Created the modern model and that electrons orbit the nucleus at fixed distances.
Isotopes- The same number of protons but different number of neutrons.
Atomic Structure
Model of the Atom
Dalton’s Model (1803)- Suggested that all matter is made of atoms and atoms cannot be split.
Thomson’s Plum Pudding Model (1897)- Discovered electrons. He created the ‘plum pudding’ model, a ball of positive charge with negatively charged electrons.

Rutherford’s nuclear Model(1909)- discovered that alpha particles can bounce back off atoms. Concluded that the atoms mass is concentrated.
Niels Bohr(1932)- Created the modern model and that electrons orbit the nucleus at fixed distances.
Isotopes- The same number of protons but different number of neutrons.
 Knowt
Knowt