
Matter and Atoms
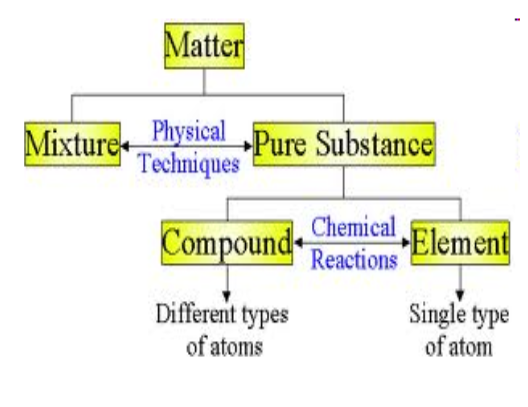
Classifying Matter:
In chemistry, we sort them based on 3 categories:
Element
Substance that cannot be decomposed into simpler substances by chemical changes
Examples: Any element on the periodic table
Compound
A substance that are made up of 2+ different elements, and can be decomposed by chemical means into simpler substances- always in the same ratio by mass
Example: Water (H2O) is always 2 hydrogen molecules and 1 oxygen molecule
Mixture
Combinations of 2+ substances where each substance is still could be separated
Homogeneous Mixtures
Mixtures that don’t contain visibly different parts
Example: Air
Heterogeneous Mixtures
Mixtures that visibly show different parts
Example: Chocolate Chip Cookies
[EXTRA note: Allotropes are different structural modifications of an element… meaning they’ll have different chemical and physical properties]
Quick Comparison:
Compounds | Mixture |
|---|---|
composed of 2+ different elements that are CHEMICALLY COMBINED | consists of 2+ different substances that are PHYSICALLY COMBINED |
can be broken down into simpler substances (elements) | could be separated by physical means |
composition is in a fixed ratio: meaning the ratio can’t be varied and the compound still being the same thing | the composition isn’t constant, meaning there could be 20% water and 80% sand but it still is sand water |
Methods of Separation:
Filtration
Used to separate suspended particles from mixtures
Example: water and sand
Distillation
Used to separate solid-liquid mixtures or liquid-liquid mixtures by using their different boiling points
Example: water and alcohol
Chromatography
Used to separate dyes and other soluble materials
Example: separation of plant pigments
Physical and Chemical Changes:
States/Phases of Matter
Solid
Definite Shape and Volume
Liquid
Indefinite Shape
Definite Volume
Gas
Indefinite Shape
Indefinite Volume
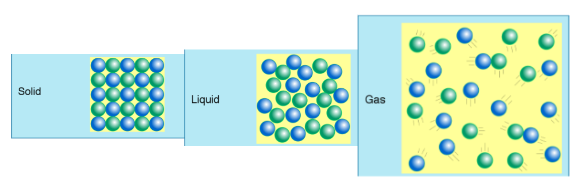
Solid | Liquid | Gas |
|---|---|---|
Incompressible: | Relatively incompressible: | Very Compressible: |
Do not conform the shape or the volume of the container: | Conform the shape of the container: | Conform the shape of the container: |
Physical Properties:
Physical Properties:
Quality of a substance that can be observed or measured WITHOUT changing the substance’s identity
Examples: Boiling point, Taste, Odor, Dissolves in water, Luster, Density, Hardness, etc.
Chemical Properties:
Any of a material’s properties that becomes evident during a chemical reaction
Basically, it can’t be determined just by viewing for touching the substance
Examples: Flammability, Ability to Rust
Physical Changes:
Some properties of a substance change but the identity of the chemical does not
PHASE CHANGES ARE PHYSICAL CHANGES !
boil, freeze, condense, break, crack
Chemical Changes:
A new chemical forms !
Structure of an Atom:
Atoms:
the smallest particle of an element that retains the chemical identity of that element
basic unit of matter
History of atoms:
Democritus: a fifth century B.C. Greek philosopher proposed that all matter was composed of tiny, indestructible particles called atoms (which is Greek for uncuttable)
Billiard Ball Model: John Dalton viewed the atom as a small solid sphere. (1803)
Plumb Pudding Model: J.J. Thompson proposed that the atom was a sphere of positive electricity with negative particles imbedded throughout after discovering the electron (1897)
It looks like a plum pudding :)
Electron Cloud Model: An atom consists of a dense nucleus composed of protons and neutrons surrounded by electrons that exist in different clouds at the various energy levels.
Discovery of Nucleus:
Ernest Rutherford: Gold foil experiment involved during the firing of radioactive particles through thin gold foils and observed them. Because only about 1/20,000 rays were deflected, this led to his theory that most of the atom was empty space
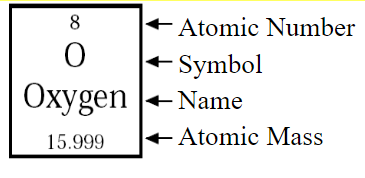
Atomic Structures using Periodic Table:
Protons, Neutrons, Electrons:
Protons:
They have a mass of approximately 1
Protons are in the nucleus
Positive charge
They determine the identity of each element: Also represents the atomic number
Neutrons:
These also have a mass of approximately 1
Neutrons are also located in the nucleus
Neutral Charge
Electrons:
These have really small mass, often times they aren’t counted
Electrons have negative charge
They’re located around the nucleus, in rings
Atomic Structures:
Atomic Number: Numbers of Protons
Atomic Mass: Numbers of Protons + Neutrons
Isotopes:
Different atoms of the same element can have different mass numbers depending on how many neutrons they have. Atoms with identical atomic numbers but different mass numbers are called isotopes.
Lewis Dot (Electron Dot) Structures:
Valence Electrons: The amount of electrons an element has on their most outermost shell
Elements with the same amount of valence electrons are typically part of the same group/family
When drawing Electron Dot Structures, we use their valence electrons
Octet Rule: States that the atoms would tend to lose/gain/share electrons in order to acquire a full set of valence electrons, which is 8 electrons
Ground State: Electrons are in their lowest energy levels, or their normal energy levels
Excited State: When heat, electricity, or light would move the electron up to different energy levels
When the electron falls back down to the ground, it releases energy and gives off light energy or a spectrum
Spectrums are unique to each element, and could be used to identify elements
When the electron becomes excited, it takes in/absorbs energy
Matter and Atoms
Classifying Matter:
In chemistry, we sort them based on 3 categories:
Element
Substance that cannot be decomposed into simpler substances by chemical changes
Examples: Any element on the periodic table
Compound
A substance that are made up of 2+ different elements, and can be decomposed by chemical means into simpler substances- always in the same ratio by mass
Example: Water (H2O) is always 2 hydrogen molecules and 1 oxygen molecule
Mixture
Combinations of 2+ substances where each substance is still could be separated
Homogeneous Mixtures
Mixtures that don’t contain visibly different parts
Example: Air
Heterogeneous Mixtures
Mixtures that visibly show different parts
Example: Chocolate Chip Cookies
[EXTRA note: Allotropes are different structural modifications of an element… meaning they’ll have different chemical and physical properties]

Quick Comparison:
Compounds | Mixture |
|---|---|
composed of 2+ different elements that are CHEMICALLY COMBINED | consists of 2+ different substances that are PHYSICALLY COMBINED |
can be broken down into simpler substances (elements) | could be separated by physical means |
composition is in a fixed ratio: meaning the ratio can’t be varied and the compound still being the same thing | the composition isn’t constant, meaning there could be 20% water and 80% sand but it still is sand water |
Methods of Separation:
Filtration
Used to separate suspended particles from mixtures
Example: water and sand
Distillation
Used to separate solid-liquid mixtures or liquid-liquid mixtures by using their different boiling points
Example: water and alcohol
Chromatography
Used to separate dyes and other soluble materials
Example: separation of plant pigments
Physical and Chemical Changes:
States/Phases of Matter
Solid
Definite Shape and Volume
Liquid
Indefinite Shape
Definite Volume
Gas
Indefinite Shape
Indefinite Volume

Solid | Liquid | Gas |
|---|---|---|
Incompressible: | Relatively incompressible: | Very Compressible: |
Do not conform the shape or the volume of the container: | Conform the shape of the container: | Conform the shape of the container: |
Physical Properties:
Physical Properties:
Quality of a substance that can be observed or measured WITHOUT changing the substance’s identity
Examples: Boiling point, Taste, Odor, Dissolves in water, Luster, Density, Hardness, etc.
Chemical Properties:
Any of a material’s properties that becomes evident during a chemical reaction
Basically, it can’t be determined just by viewing for touching the substance
Examples: Flammability, Ability to Rust
Physical Changes:
Some properties of a substance change but the identity of the chemical does not
PHASE CHANGES ARE PHYSICAL CHANGES !
boil, freeze, condense, break, crack
Chemical Changes:
A new chemical forms !
Structure of an Atom:
Atoms:
the smallest particle of an element that retains the chemical identity of that element
basic unit of matter
History of atoms:
Democritus: a fifth century B.C. Greek philosopher proposed that all matter was composed of tiny, indestructible particles called atoms (which is Greek for uncuttable)
Billiard Ball Model: John Dalton viewed the atom as a small solid sphere. (1803)
Plumb Pudding Model: J.J. Thompson proposed that the atom was a sphere of positive electricity with negative particles imbedded throughout after discovering the electron (1897)
It looks like a plum pudding :)
Electron Cloud Model: An atom consists of a dense nucleus composed of protons and neutrons surrounded by electrons that exist in different clouds at the various energy levels.
Discovery of Nucleus:
Ernest Rutherford: Gold foil experiment involved during the firing of radioactive particles through thin gold foils and observed them. Because only about 1/20,000 rays were deflected, this led to his theory that most of the atom was empty space
Atomic Structures using Periodic Table:
Protons, Neutrons, Electrons:
Protons:
They have a mass of approximately 1
Protons are in the nucleus
Positive charge
They determine the identity of each element: Also represents the atomic number
Neutrons:
These also have a mass of approximately 1
Neutrons are also located in the nucleus
Neutral Charge
Electrons:
These have really small mass, often times they aren’t counted
Electrons have negative charge
They’re located around the nucleus, in rings
Atomic Structures:
Atomic Number: Numbers of Protons
Atomic Mass: Numbers of Protons + Neutrons

Isotopes:
Different atoms of the same element can have different mass numbers depending on how many neutrons they have. Atoms with identical atomic numbers but different mass numbers are called isotopes.
Lewis Dot (Electron Dot) Structures:
Valence Electrons: The amount of electrons an element has on their most outermost shell
Elements with the same amount of valence electrons are typically part of the same group/family
When drawing Electron Dot Structures, we use their valence electrons
Octet Rule: States that the atoms would tend to lose/gain/share electrons in order to acquire a full set of valence electrons, which is 8 electrons
Ground State: Electrons are in their lowest energy levels, or their normal energy levels
Excited State: When heat, electricity, or light would move the electron up to different energy levels
When the electron falls back down to the ground, it releases energy and gives off light energy or a spectrum
Spectrums are unique to each element, and could be used to identify elements
When the electron becomes excited, it takes in/absorbs energy
 Knowt
Knowt