
12-ISA: Redox Reactions & Electrochemical Cells
Sheet 1 (p. 7)
Key Information
Oxidation reduction (redox) reactions: reactions in which oxidation numbers/states change
Oxidation numbers/states: either real charges or assigned charges which help us to keep track of electron transfer
Oxidation cannot occur without reduction
In a redox reaction, the substance which is oxidized: contains atoms which increase in oxidation number
Oxidation is associated with electron loss
LEO (Loss of electrons, oxidation)
In a redox reaction, the substance which is reduced: contains atoms which decrease in oxidation number during the reaction
Reduction is associated with electron gain
GER (Gain of electrons, reduction)
LEO the lion says GER
Oxidizing agent: a substance that oxidizes something else but it itself is reduced
Reducing agent: a substance which reduces something else but it itself is oxidized
Disproportion reaction: reaction in which the same element is both oxidized and reduced
Rules, how to assign oxidation numbers
The oxidation number of any pure element: zero
e.g. oxidation number of H in H₂ is 0
The oxidation number of a monoatomic ion: equal to its charge
e.g. oxidation number for Mg in Mg²⁺ is +2
The sum of oxidation numbers in a compound: zero if neutral, or equal to the charge if an ion
The oxidation number of of alkali metals (group 1) in compounds is: +1
The oxidation number of alkaline earth metals (group 2) in compounds is: +2
The oxidation number of Fluorine (F) is: -1 in all of its compounds
The oxidation number of H is: +1 in most compounds. Exceptions are H₂ where H= 0 and the ionic hydrides where H= -1 (e.g. NaH)
The oxidation number of O is: -2 in most compounds. Exceptions are O₂ where O= 0 and peroxides where O = -1 (e.g. H₂O₂)
Sheet 2 (p. 12)
Important additional hints for finding oxidation numbers/states
If the element being considered is diatomic (appears alone): the oxidation state of each atom is zero
Only HOFBrINCl elements are diatomic
The oxidation state of elements in ionic compounds is: the same as its ion charge. Ions are not diatomic
Individual oxidations states must add up to zero for a neutral compound, or to the charge of the ion if the substance is charged
e.g. In NH₄¹⁺, the oxidation states on N and H must add up to +1
Always use the anion’s charge to find the cation’s charge (oxidation state) in an ionic compound
e.g. PbS₂
Since the sulfur ion has a charge of -2 and there are two of them, the total negative charge is -4 and so if there is only one lead ion, it must have a charge/oxidation state of +4 (to get it to 0)
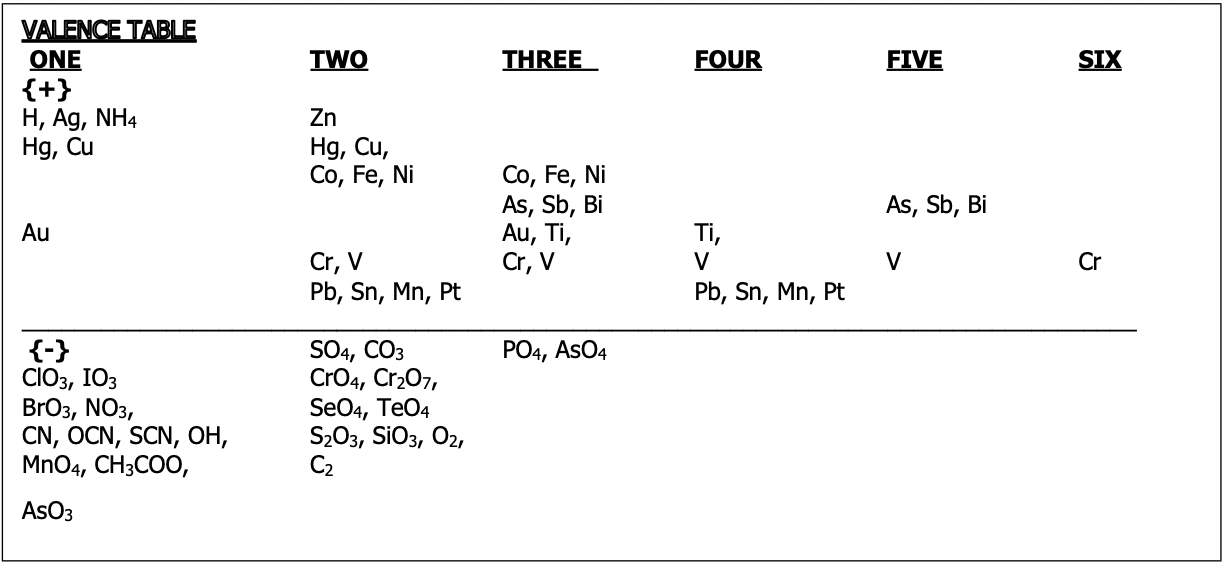
Helpful to refer to the periodic table and the valence table shown below for anion charges
Adding/removing oxygen atoms from a polyatomic does affect its name, however it doesn’t affect its overall charge
e.g. SO₄ and SO₃ have the same charge of -2
Valence Table
The top half of the table has the cations and the bottom has the anions
The column numbers correspond to the magnitude of the charge
Sheet 3 (p. 14)
Balancing redox reactions, oxidation number method
Must also balance for ion charge as well as atoms
Oxidation numbers offer a systematic method for balancing redox reactions, and the method is based on the fact that the number of electrons released by the oxidized substance must equal the number gained by the reduced substance. Once these are balanced, the rest of the elements will easily balance.
Example 1
Step 1): assign oxidation numbers to all atoms in the equation
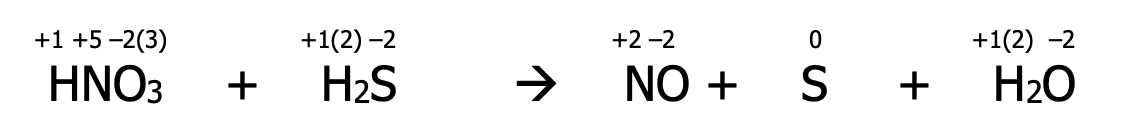
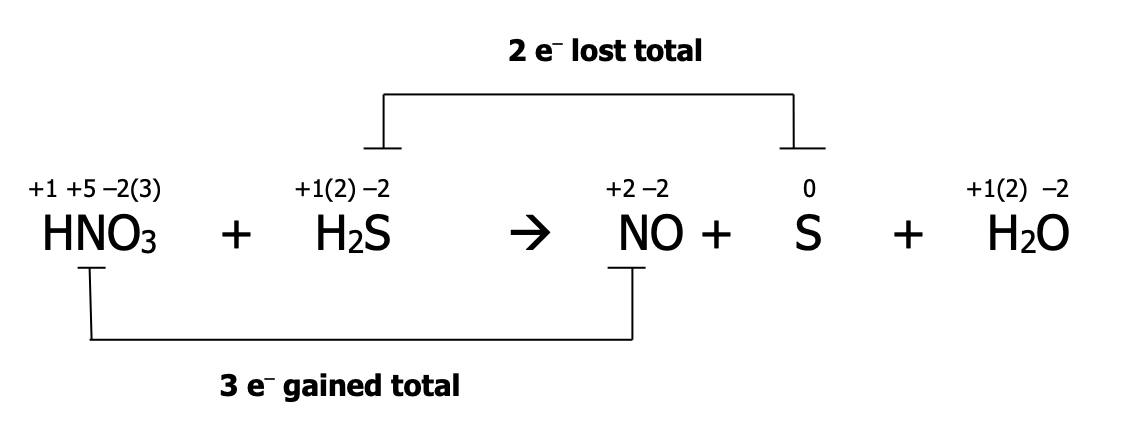
Step 2): identify the atoms which have been oxidized and reduced. These atoms will have had a change in their oxidation number. Draw a line to connect them. If they’re not already balanced, add coefficients to balance them. State the total number of electrons lost & gained in each case
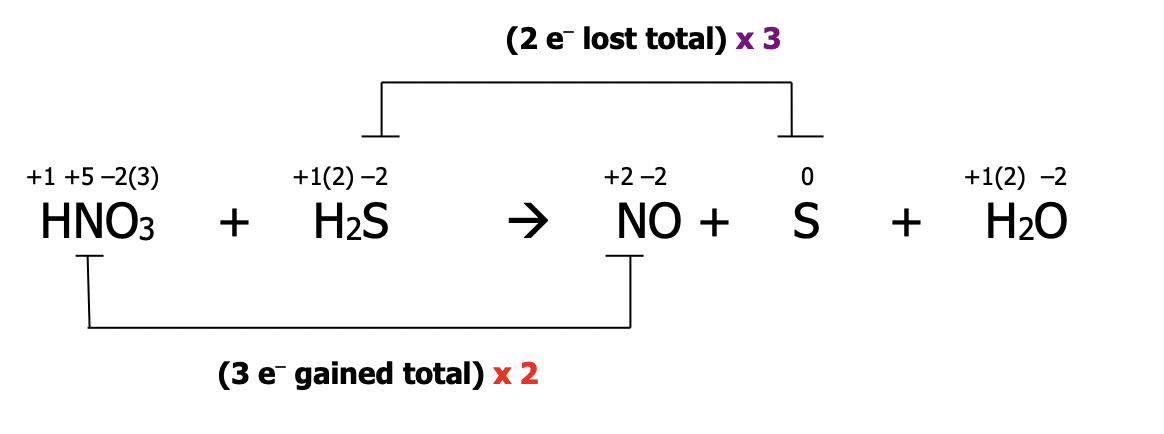
Step 3): balance electrons to make sure the number lost = the number gained by multiplying by the appropriate factor
Step 4): Balance the redox participants by putting the multiplying factors from step 3) into the main equation
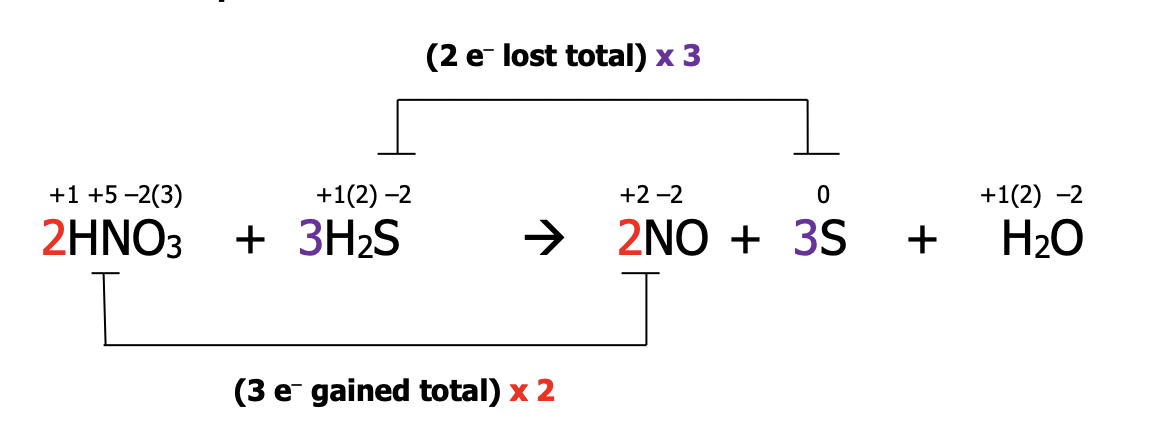
Step 5): Balance all other atoms by inspection
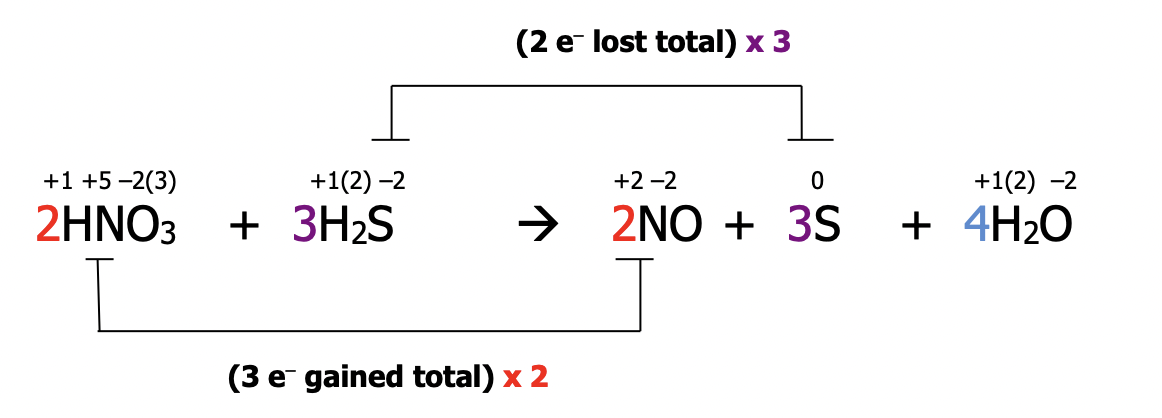
Step 6): Rewrite the completed balanced equation without all the extra numbers
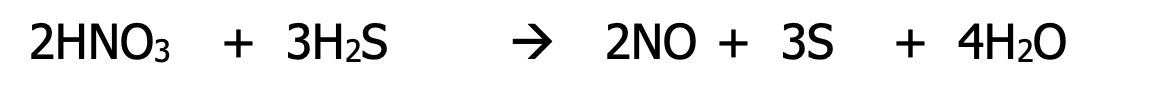
Sheet 4 (p. 27)
Now we will be given a skeleton equation to balance
In a skeleton equation, only the oxidized and reduced species are shown. You have to add the necessary conditions (acid or base) but the question will always specify which
Example: balance the following skeleton equation using the oxidation number method under acidic conditions
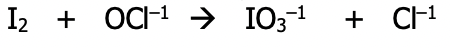
Step 1): Follow steps above 1-4
Step 1): assign oxidation numbers to all atoms in the equation
Step 2): identify the atoms which have been oxidized and reduced. These atoms will have had a change in their oxidation number. Draw a line to connect them. If they’re not already balanced, add coefficients to balance them. State the total number of electrons lost & gained in each case
Step 3): balance electrons to make sure the number lost = the number gained by multiplying by the appropriate factor
Step 4): Balance the redox participants by putting the multiplying factors from step 3) into the main equation
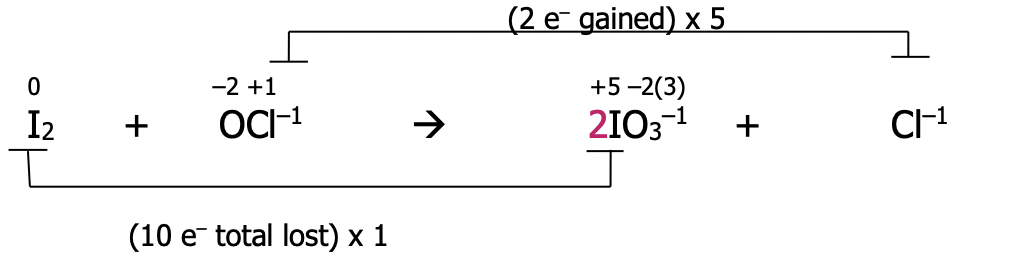
Step 2): At the inspection step, balance oxygen by adding water and hydrogen by adding H¹⁺. Look at both sides of the equation for atoms of hydrogen
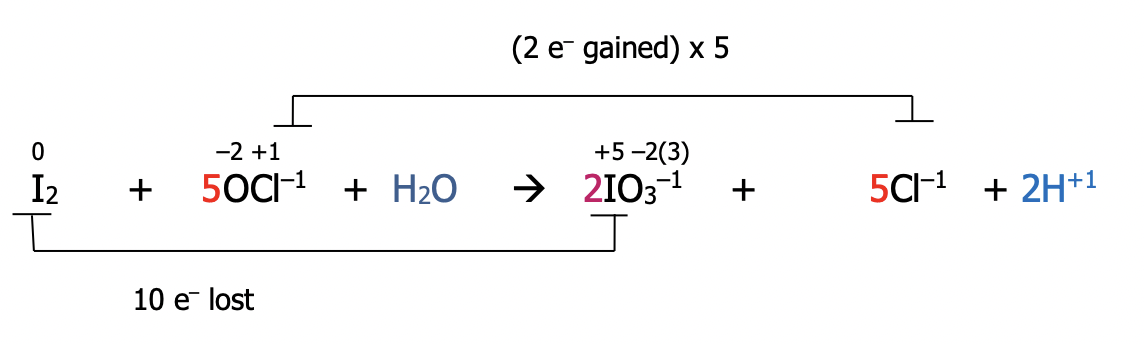
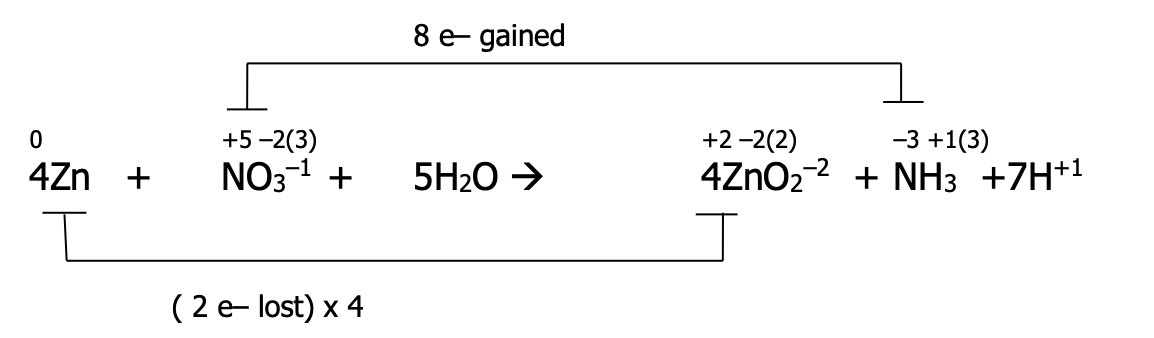
Example: Balance the following redox reaction using the oxidation number method under basic conditions

Step 1): Follow all previous steps, as if the reaction occurs in acid
Step 1): Follow steps 1-4
Step 1): assign oxidation numbers to all atoms in the equation
Step 2): identify the atoms which have been oxidized and reduced. These atoms will have had a change in their oxidation number. Draw a line to connect them. If they’re not already balanced, add coefficients to balance them. State the total number of electrons lost & gained in each case
Step 3): balance electrons to make sure the number lost = the number gained by multiplying by the appropriate factor
Step 4): Balance the redox participants by putting the multiplying factors from step 3) into the main equation
Step 2): At the inspection step, balance oxygen by adding water and hydrogen by adding H¹⁺. Look at both sides of the equation for atoms of hydrogen
Step 2): Now add hydroxide ions to each side equal to the number of hydrogen ions. Adding the same value to both sides of an equation maintains the equality
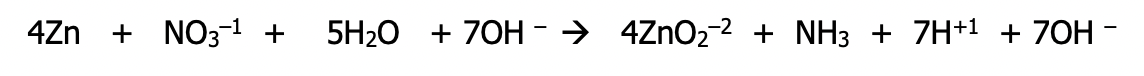
Step 3): Simplify by recognizing that 1 H⁺
Sheet 5 (p. 33)
The half-cell method of balancing redox equations offers an alternate way to achieve balance
Half-cell steps vs. oxidation method steps
Difference in order of operation
The other atoms are balanced first and the electrons are balanced last in the half-cell method
Balancing reactions in acidic solution
Example: Balance using the half-cell method in acidic medium
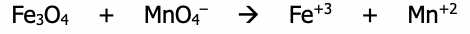
Step 1): Divide the equation into 2 possible half reactions - it isn’t needed to know which half is oxidation and which half is reduction at this point
 Step 2): Balance each half reaction separately (balance all elements besides H and O first)
Step 2): Balance each half reaction separately (balance all elements besides H and O first)
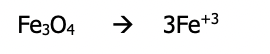 Step 3): If needed, balance the O atoms by adding H₂O to the side needing more O
Step 3): If needed, balance the O atoms by adding H₂O to the side needing more O
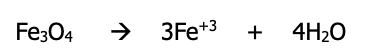 Step 4): Balance the H atoms by adding H⁺ to the side that needs it
Step 4): Balance the H atoms by adding H⁺ to the side that needs it
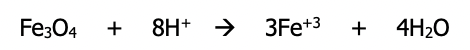 Step 5): Balance for charge (the total charge on the left side must be ===== to total charge on the right side) by adding electrons to the side with greater overall positive charge
Step 5): Balance for charge (the total charge on the left side must be ===== to total charge on the right side) by adding electrons to the side with greater overall positive charge
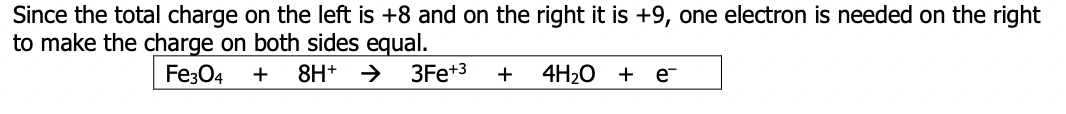 This equation is the actual oxidation half reaction where electrons are released
This equation is the actual oxidation half reaction where electrons are released
Step 6): Repeat the above steps to balance the other half reaction. Since the one above turned out to be an oxidation, the next one should be a reduction (electrons required). This equation will be the actual reduction half reaction
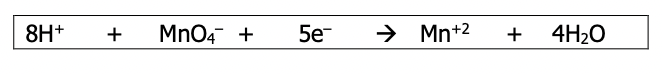 Step 7): Adjust the coefficients of the two balanced half-reactions such that the number of electrons lost is = to the number of electrons gained
Step 7): Adjust the coefficients of the two balanced half-reactions such that the number of electrons lost is = to the number of electrons gained
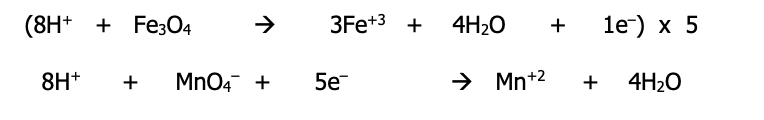 Step 8): Add the 2 reactions to obtain the balanced equation for the redox reaction. Simplify H⁺ and H₂O if needed
Step 8): Add the 2 reactions to obtain the balanced equation for the redox reaction. Simplify H⁺ and H₂O if needed
Since the number of electrons lost and gained are now equal, they should cancel out
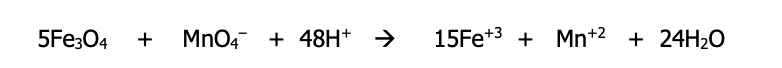
Sheet 6 (p. 40)
Using half-cell method for basic solution
As with the oxidation method, in basic solution you follow the exact same method for acid and then add OH⁻ to both sides to adjust to base
Example: Balance using the half-cell method in basic medium
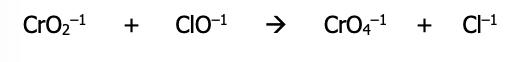 Step 1): Balance each half reaction as if it occurs in acidic solution using the steps described earlier
Step 1): Balance each half reaction as if it occurs in acidic solution using the steps described earlier
Step 1): Divide the equation into 2 possible half reactions - it isn’t needed to know which half is oxidation and which half is reduction at this point
Step 2): Balance each half reaction separately (balance all elements besides H and O first)
Step 3): If needed, balance the O atoms by adding H₂O to the side needing more O
Step 4): Balance the H atoms by adding H⁺ to the side that needs it
Step 5): Balance for charge (the total charge on the left side must be = to total charge on the right side) by adding electrons to the side with greater overall positive charge
Step 6): Repeat the above steps to balance the other half reaction. Since the one above turned out to be an oxidation, the next one should be a reduction (electrons required). This equation will be the actual reduction half reaction
Step 7): Adjust the coefficients of the two balanced half-reactions such that the number of electrons lost is = to the number of electrons gained
Step 8): Add the 2 reactions to obtain the balanced equation for the redox reaction. Simplify H⁺ and H₂O if needed
Since the number of electrons lost and gained are now equal, they should cancel out
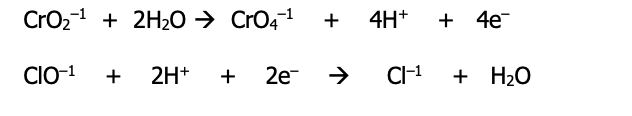 Step 2): Neutralize the H⁺ ions by adding an equal number of OH⁻ ions to both sides of the equation (H⁺ + OH⁻ = H₂O) & simplify
Step 2): Neutralize the H⁺ ions by adding an equal number of OH⁻ ions to both sides of the equation (H⁺ + OH⁻ = H₂O) & simplify
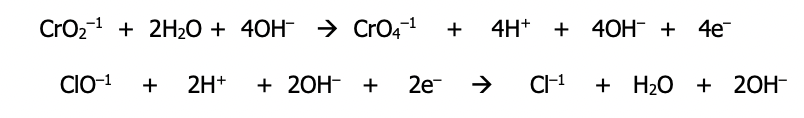 Step 3): Remember that H⁺ + OH⁻ = H₂O & simplify
Step 3): Remember that H⁺ + OH⁻ = H₂O & simplify
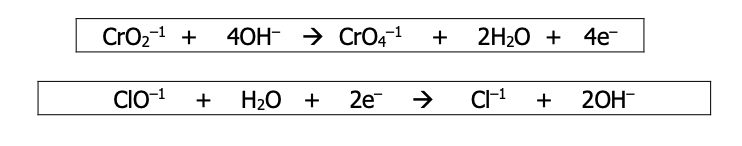 Step 4): Adjust the coefficients of the two balanced half reactions such that the number of electrons lost equals the number of electrons gained. Add the two reactions and simplify further if needed
Step 4): Adjust the coefficients of the two balanced half reactions such that the number of electrons lost equals the number of electrons gained. Add the two reactions and simplify further if needed
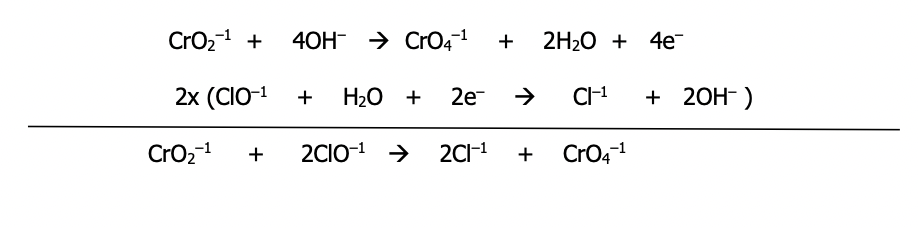
Sheet 7 (p. 47)
Galvanic cells (aka Voltaic or Electrochemical. cells) harness the potential energy of a redox reaction and use it to do useful work (e.g. a 1.5 V battery is a Galvanic cell)
In all redox reactions, the reducing agent (which loses electrons) passes electrons to the oxidizing agent (which gains electrons)
A single displacement reaction is a simple example of a redox reaction that can be used in an electrochemical cell
e.g.
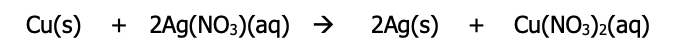
Anytime a single displacement reaction is done, the metal higher on the activity series will lose electrons (and be oxidized) and the metal below it will thus gain electrons (and be reduced)
Activity series: 
If the 2 half reactions are physically separated and connected by a conductor (a wire), then the electrons can only be transferred through the wire from one element to another
Diagram of a Galvanic/electrochemical cell:
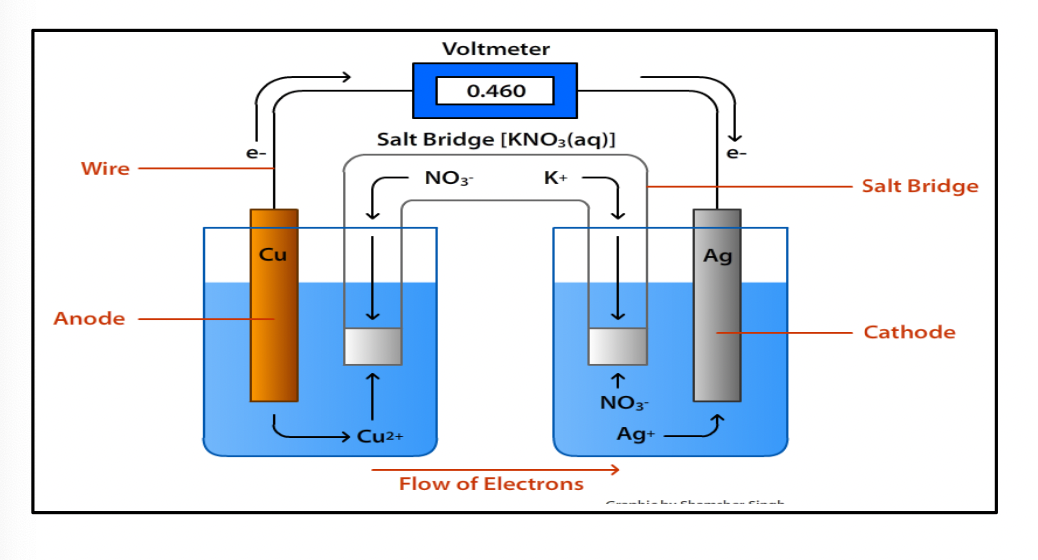
All of the substances involved in the reaction must be present in the electrochemical cell assembly however the oxidation & reduction reactions happen in their own compartment: referred to as half-cells
In the diagram’s case above, each half cell contains a beaker of an ionic solution, where the cation is the metal ion involved in the reaction and a strip of the same metal element partially immersed in the solution. This strip of metal is called an electrode
For this example, there is a piece of cooper metal immersed in a copper (II) nitrate solution (usually 1.0 M solutions are used) and a piece of silver metal immersed in a silver nitrate solution. Nitrate is a good anion to pair with each cation because all cations are soluble with it
The cathode: the electrode where reduction takes place
The anode: the electrode where oxidation takes place
The anode is where the electrons originate. Because of this, the anode is more negative relative to the cathode & so the anode is labelled as the negative electrode and the cathode as the positive electrode
This corresponds to the negative and positive terminals of a cell or battery
The redox used in the above example is a single displacement but any reaction can form the basis of an electrochemical cell
In many redox reactions (but not all) the electrodes are involved in the reaction. The electrodes are only involved if the reaction includes solid metal atoms that become metal cations or vice versa
When metal atoms of the anode loses electrons to become cations which enter/dissolve into the solution, the solution becomes positively charged and the mass of the anode decreases. It decreases because the neutral atoms have become ions and have left the electrode for the solution. This is illustrated by the following net (the spectator anion nitrate has been removed) oxidation of half reaction

At the cathode, ions in the solution pick up the incoming electrons & are reduced to become neutral metal atoms. They deposit on the electrode so its mass increases. The solution begins to become negatively charged because the cations are consumed from the solution (the anions that are in the solution will now be greater in number)
This is illustrated by the following net (the spectator anion has been removed) reduction half reaction
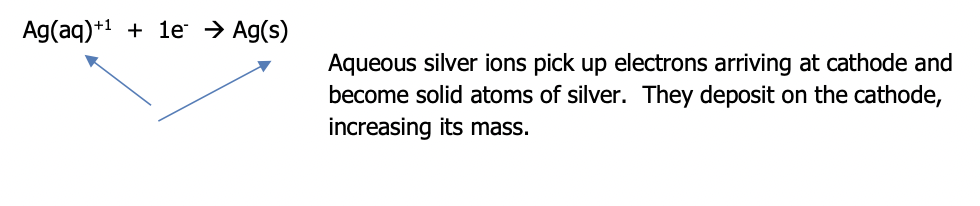
The net redox reaction is the sum of the two half reactions (note that the silver half reaction will need to be multiplied by 2 to get the electrons lost to those gained)
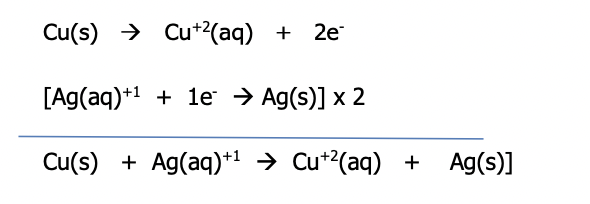 Reconsider the diagram of the Galvanic cell
Reconsider the diagram of the Galvanic cell
Salt Bridges
There is a secondary connection between the half-cells called a salt bridge
Salt bridge: needed to connect the two half cells to allow ions to migrate back and forth between the half cell solutions.
Recall that the solution containing the anode becomes positively charged over time as metal atoms turn into cations and enter the solutions whereas the solution containing the cathode becomes negatively charged as anions will be in greater number since cations are depleted by the reaction
This building charge in each beaker will stop the flow of electrons through the wire from the anode to the cathode. e.g.) Since the copper ion concentration builds, these cations attract the electrons back faster than they can travel through the wire to the other side to be picked up by silver ions & so the current flow stops
To keep the driving force on, the electrons to go from the anode to the cathode, the electrical neutrality of each solution must be maintained
It can be as simple as a piece of filter paper soaked in soluble salt solution. NaNO₃ and KNO₃ are usually good choices for they tend not to interfere with the reaction & are soluble
Anions always flow towards the anode
The solution is becoming positive so it will attract anions to achieve neutrality
Cations always flow towards the cathode
The solution is becoming negative so it will attract cations to achieve neutrality
Remember that the sign assigned to the anode is negative (-) which is different than the charge building up in the solution, which is positive (+) –– the same is true for the cathode
In a Galvanic cell, electrons always flow from the anode to the cathode through the wire, anions flow to the anode half cell through the salt bridge and cations flow to the cathode half cell through the salt bridge
Inert Electrodes
If a half reaction does not include a solid metal and either the oxidation or reduction half reactions (or both) happen within the solution only or involve gases a metal electrode is still needed to allow the electrons to travel from one hand cell to the other
Platinum (a very inert metal found at the bottom of the activity series) is usually the ideal choice as the electrode since it will not interfere with ongoing reactions
The platinum electrode will act as a conductor to allow the electrons released from oxidation to trace to the cathode (which could possibly be another platinum electrode if the reduction occurs in solution or involves gases)
Graphite is an allotrope of carbon and can also be used as an inert electrode
Cell Line Notation
Cell line notation is a special notation used to represent electrochemical cells that indicates the redox reaction occurring
Rules:
The anode half cell is described first followed by the cathode half cell
Within a given half cell, the reactants are specified first and the products last – the description of the oxidation reaction is first and the reduction reaction is last – when you read it, your eyes move in the direction of electron flow – spectator ions aren’t usually included
A single vertical line is drawn between 2 chemical species that are in different phases but in physical contact with each other (e.g. solid electrode | aqueous solution)
A double vertical line (||) represents a salt bridge or porous membrane separating the individual half cells
The phase of each chemical (i.e. s, l, g, aq) is shown in brackets – if the electrolytes in the cells aren’t at STP it must be included in brackets with the phase notation, if not noted then the electrolytes are assumed to be at standard conditions
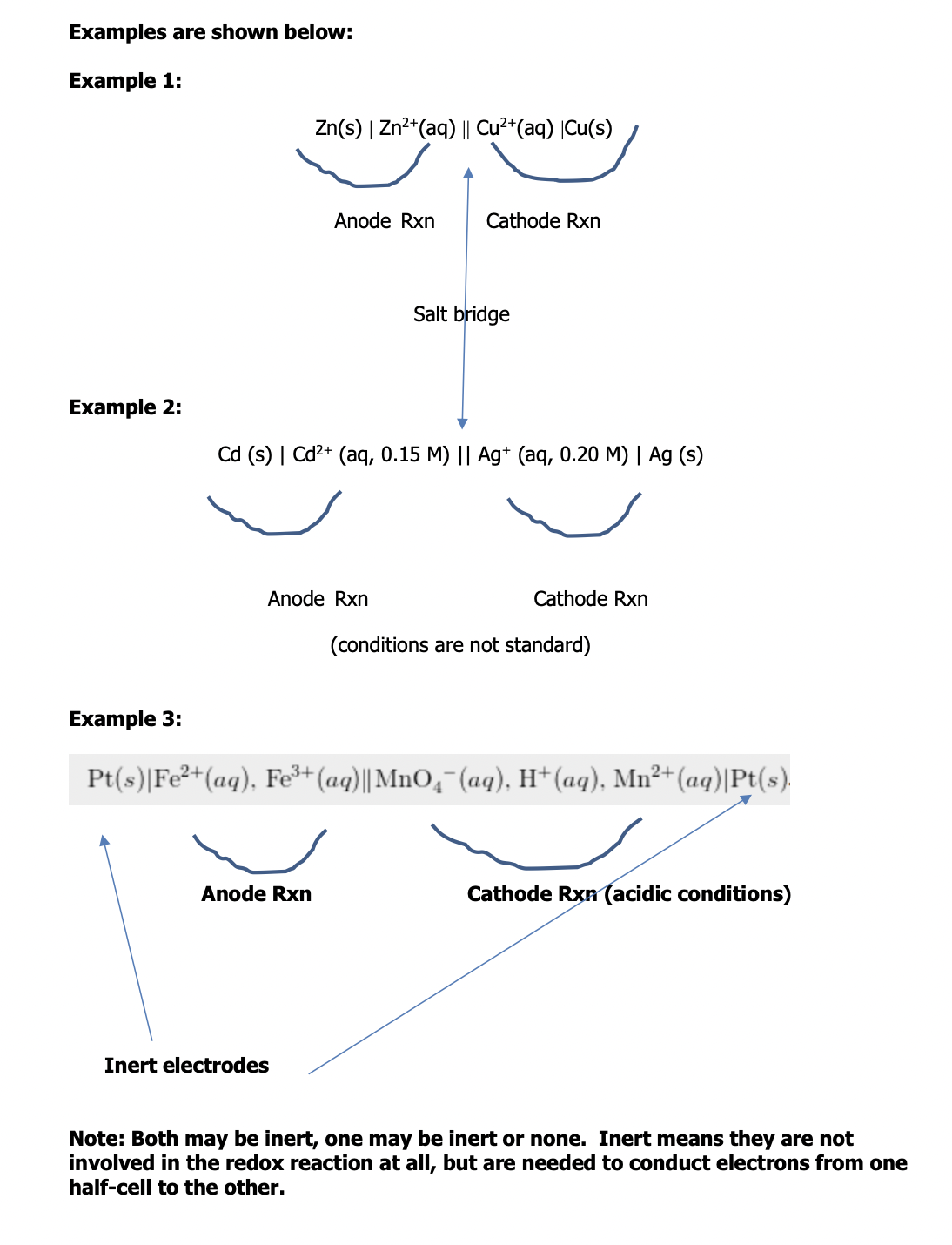
Sheet 8 (p. 54)
Standard Electrode Potentials
In a galvanic cell, electrons are produced at the anode (oxidation) and picked up at the cathode (reduction)
Means that the anode will will be more negative than the cathode
Anode is often labelled negative & the cathode is labelled + but the cathode is actually just less negative
Voltmeter: compares the difference in this charge build up between the electrodes
The greater the difference, the greater the voltage reading (aka potential difference or electromotive force)
It essentially measures the “electric pressure” that exists between the anode & cathode or the propensity for the electrons to move from the anode to the cathode
When the substance being oxidized has a very high tendency to lose electrons (strong reducing agent) and the one being reduced has a very high tendency to gain (strong oxidizing agent) – there will be a greater potential difference between them and thee combination will result in a higher voltage reading than if they have more similar oxidation/reduction potentials
A waterfall is a good analogy – the greater the drop, the greater the gravitational potential difference
The voltage or potential difference of an electrochemical cell under standard conditions has the symbol Eºcell
Impossible to measure the potential or voltage of a half cell alone – to get around this, there is a standard that everything is compared to, this is called the hydrogen half cell
This reference half cell consists of a Pt electrode in a solution containing 1 M H⁺ into which H₂ gas is added at a constant pressure of 1 atm and is assigned a potential of 0 V
The reaction is:
H⁺ (aq) + 2 e⁻ → H₂ (g) Eºr = 0 V
The cell potential measured for a given half-cell connected to hydrogen half-cell is reported as the standard half-cell potential of the substance since the hydrogen value is assigned 0 V
By convention, standard reduction potentials (symbolized by Eºr) are reported rather than both reduction and oxidation potentials - these potentials are a measure of the tendency of a given half-cell to gain electrons from the hydrogen half-cell
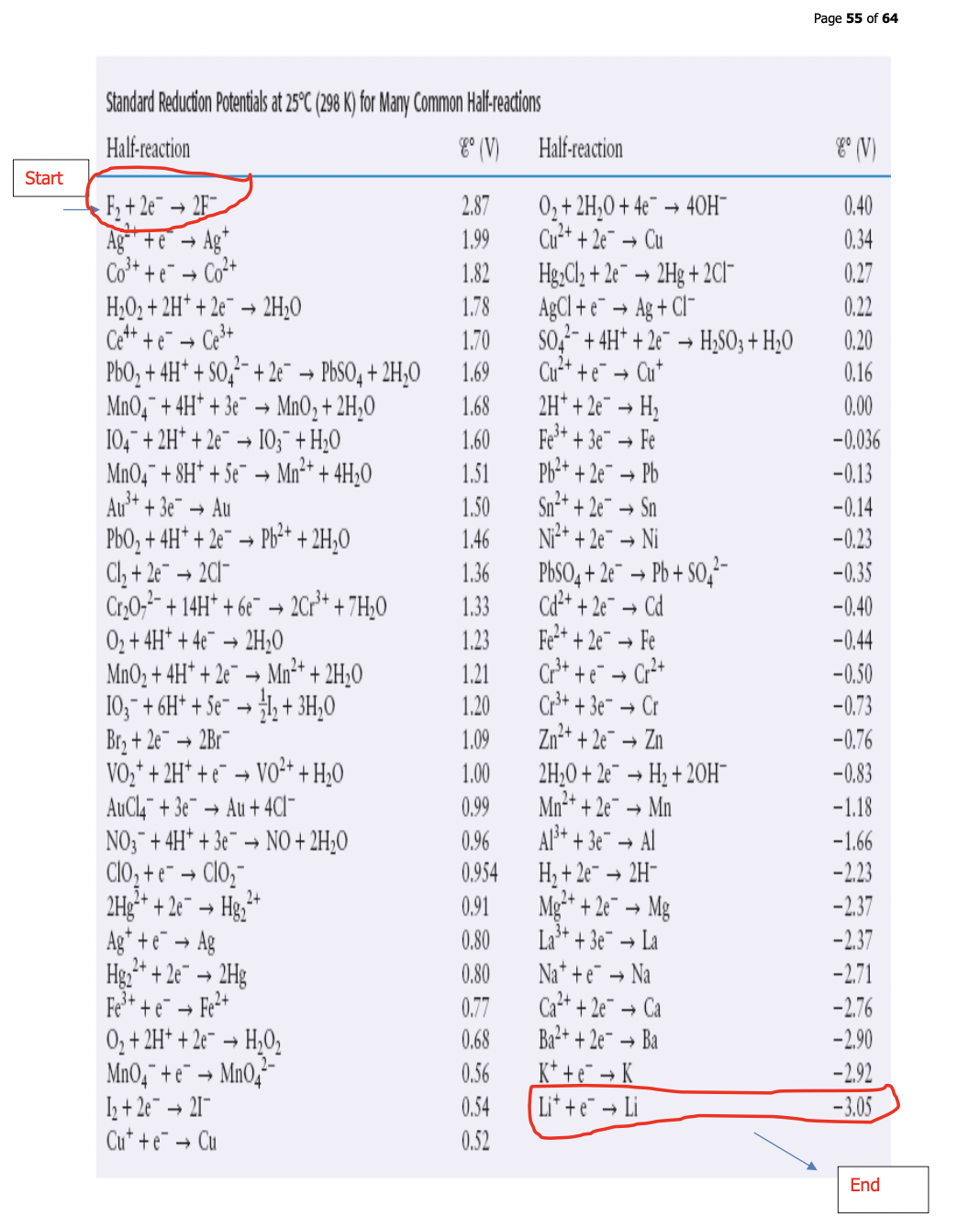
Sheet 9 (p. 58)
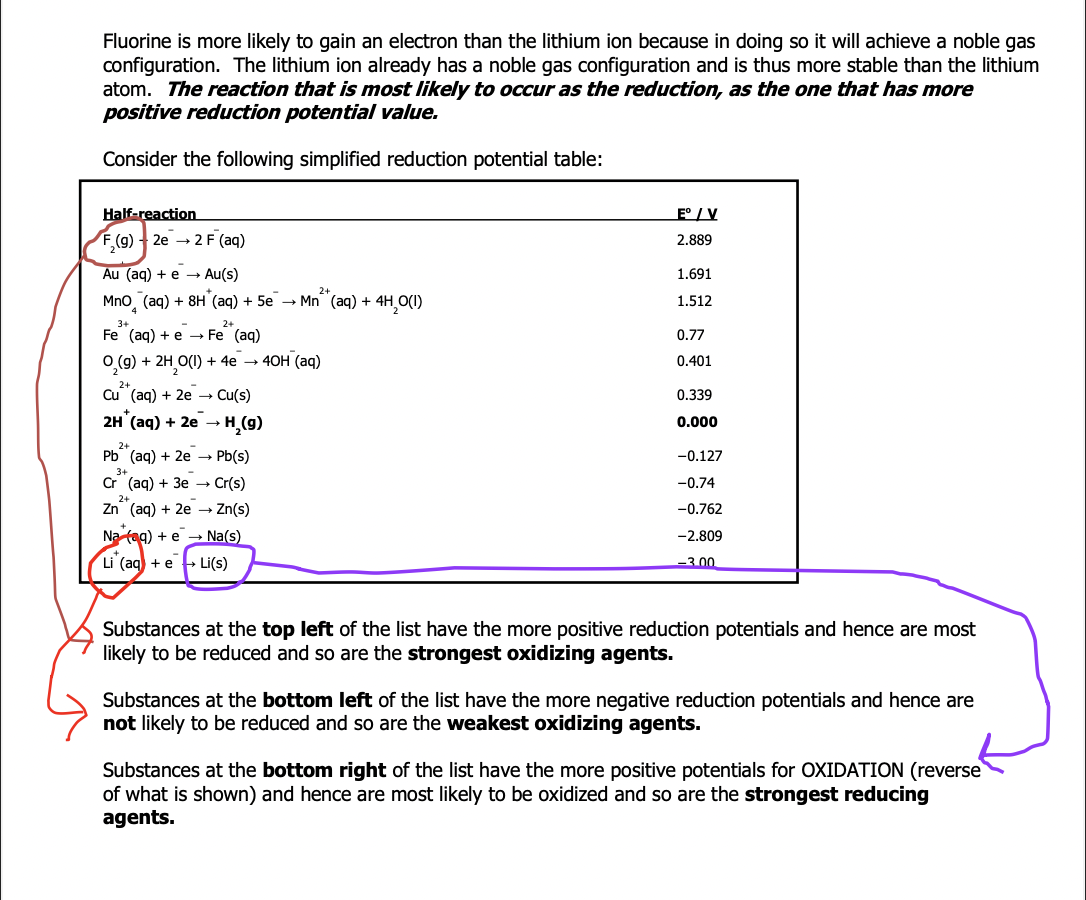
Spontaneity rule: a redox reaction will be spontaneous if the oxidizing agent is above the reducing agent on a table of reduction potentials and the overall cell potential will be positive
The overall cell potential (Eºcell) can be obtained by adding the potentials pf the reduction (Eºr) and of the oxidation had reactions (Eºox)
Reverse the sign of the potential when flipping the reduction equation to get the oxidation equation

If the half reaction is multiplied by a factored to make the number of electrons lost equal that gained – do not multiply the potential as it isn’t affected by the number of electrons transferred (these are separate ideas and quantities)
Example: use a redox table to determine if a spontaneous redox reaction can occur. If so, find the most spontaneous redox reaction that will occur, write the equations for all relevant reactions and the net reaction and calculate the cell potential
Tin (II) bromide is poured into a solution of acidified sodium dichromate
Step 1: list all entities present
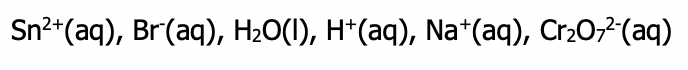
- Aqueous ionic compounds must be split into their ions
- Covalent compounds remain intact even when aqueous unless they are acids which also form ions
- Do not forget to list water if conditions are aqueous
- Do not forget to list H⁺ if conditions are acidic or OH⁻ if conditions are basic
- Bromine is the diatomic element, but bromide is the ion and the same applies to the other halogens
Step 2: classify entities as either oxidizing agents (left side of the equation in a redox table) or reducing agents (right side of the equation in redox table)
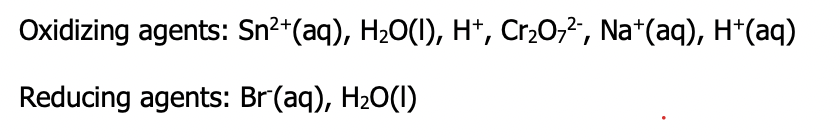
- Go down the table and highlight/circle each entity present in solution
- Circle only the exact entity to have no matter which side it appears on
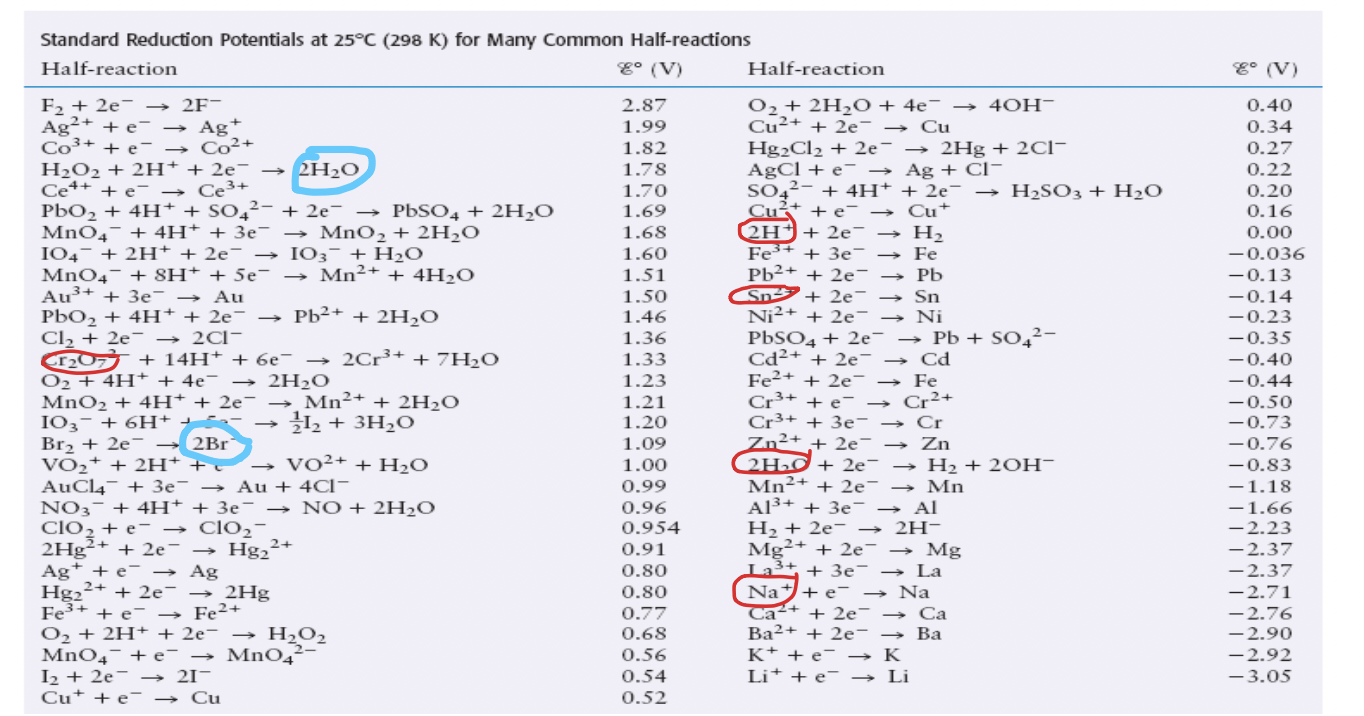
- Only circle a given substance, if all the reactants are present in the mixture, otherwise the reaction can not occur and so is not a possibility (products irrelevant).
-- In this example, there are other reactions that contain water, for instance, besides the ones that were selected, but they could not be used because they contained additional reactants that were not present in the original mixture and so could not occur.
- If your substance is on the reactant side, the reaction can only occur in the forward direction and so the substance can potentially be reduced and is an oxidizing agent.
- If your substance appears on the product side, the reaction can only occur in the reverse direction, and so the substance can only be oxidized and is a reducing agent.
Step 3: identify the strongest oxidizing agent (closest to top left) and strongest reducing agent (closest to bottom right)
-- consider all substance you circle on the left side of each reaction and choose the one closes to the top (highest one)- it is most likely to be reduced (strongest oxidizing agent)
-- consider all substances you circled on the right side of each reaction, choose the one closest to the bottom (lowest one) - it is most likely to be oxidized (strongest reducing agent)
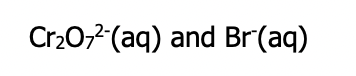
Step 4: Apply spontaneity rule
Since the strongest oxidizing agent is above the strongest reducing agent a spontaneous redox reaction will occur & by picking the strongest oxidizing and strongest reducing agents, this combo will produce the greater potential difference thus being the most spontaneous
- The same substance can appear in more than 1 reaction or be both oxidized & reduced
-- never use a reaction unless you have all reactants included in that reaction in the mixture.
--- If the reaction occurs as a reduction, the reactants are those substances on the left side of the equations in the redox table. If the reaction occurs as an oxidation, the reactants are those substances on the right side of the equations in the redox table. If a reaction involving nitrate ion requires acidic conditions and none are present, for example, that reaction cannot occur, even if the nitrate ion is the strongest OA. Use the next strongest OA, provided all reactants are found in the mixture.
Step 5: write the half-reactions and the net reaction
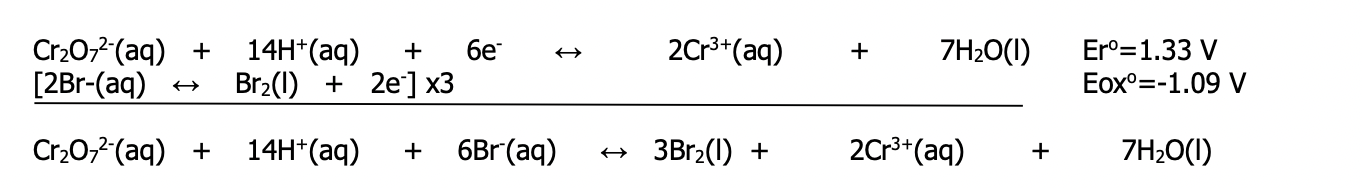
-- Make sure the number of electrons lost = gained by multiplying the entire equation by the appropriate factor
-- Do not multiply reduction potentials (Eºr) or oxidation potentials (Eºox) by this factor
Step 6: Calculate the standard cell potential

-- Use the formula (Ecell = Ecathode - Eanode) without changing any of the signs or change the sign of the reduction potential that actually occurs as an oxidation and add the two values
Do not do both or the answer will be wrong
--- In the above example, the sign was reversed from what was shown in the table for the oxidation reaction and so the values were simply added to obtain the cell potential. If using the formula, do not change the sign and use the formula as the (–) in front of the Eanode will do this for you
12-ISA: Redox Reactions & Electrochemical Cells
Sheet 1 (p. 7)
Key Information
Oxidation reduction (redox) reactions: reactions in which oxidation numbers/states change
Oxidation numbers/states: either real charges or assigned charges which help us to keep track of electron transfer
Oxidation cannot occur without reduction
In a redox reaction, the substance which is oxidized: contains atoms which increase in oxidation number
Oxidation is associated with electron loss
LEO (Loss of electrons, oxidation)
In a redox reaction, the substance which is reduced: contains atoms which decrease in oxidation number during the reaction
Reduction is associated with electron gain
GER (Gain of electrons, reduction)
LEO the lion says GER
Oxidizing agent: a substance that oxidizes something else but it itself is reduced
Reducing agent: a substance which reduces something else but it itself is oxidized
Disproportion reaction: reaction in which the same element is both oxidized and reduced
Rules, how to assign oxidation numbers
The oxidation number of any pure element: zero
e.g. oxidation number of H in H₂ is 0
The oxidation number of a monoatomic ion: equal to its charge
e.g. oxidation number for Mg in Mg²⁺ is +2
The sum of oxidation numbers in a compound: zero if neutral, or equal to the charge if an ion
The oxidation number of of alkali metals (group 1) in compounds is: +1
The oxidation number of alkaline earth metals (group 2) in compounds is: +2
The oxidation number of Fluorine (F) is: -1 in all of its compounds
The oxidation number of H is: +1 in most compounds. Exceptions are H₂ where H= 0 and the ionic hydrides where H= -1 (e.g. NaH)
The oxidation number of O is: -2 in most compounds. Exceptions are O₂ where O= 0 and peroxides where O = -1 (e.g. H₂O₂)
Sheet 2 (p. 12)
Important additional hints for finding oxidation numbers/states
If the element being considered is diatomic (appears alone): the oxidation state of each atom is zero
Only HOFBrINCl elements are diatomic
The oxidation state of elements in ionic compounds is: the same as its ion charge. Ions are not diatomic
Individual oxidations states must add up to zero for a neutral compound, or to the charge of the ion if the substance is charged
e.g. In NH₄¹⁺, the oxidation states on N and H must add up to +1
Always use the anion’s charge to find the cation’s charge (oxidation state) in an ionic compound
e.g. PbS₂
Since the sulfur ion has a charge of -2 and there are two of them, the total negative charge is -4 and so if there is only one lead ion, it must have a charge/oxidation state of +4 (to get it to 0)
Helpful to refer to the periodic table and the valence table shown below for anion charges
Adding/removing oxygen atoms from a polyatomic does affect its name, however it doesn’t affect its overall charge
e.g. SO₄ and SO₃ have the same charge of -2
Valence Table
The top half of the table has the cations and the bottom has the anions
The column numbers correspond to the magnitude of the charge

Sheet 3 (p. 14)
Balancing redox reactions, oxidation number method
Must also balance for ion charge as well as atoms
Oxidation numbers offer a systematic method for balancing redox reactions, and the method is based on the fact that the number of electrons released by the oxidized substance must equal the number gained by the reduced substance. Once these are balanced, the rest of the elements will easily balance.
Example 1
Step 1): assign oxidation numbers to all atoms in the equation
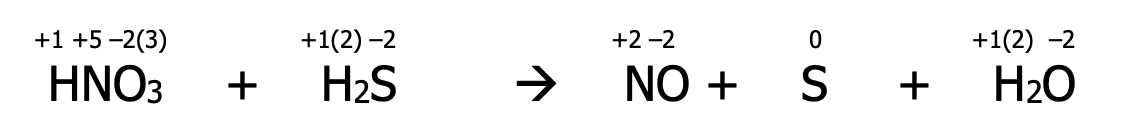
Step 2): identify the atoms which have been oxidized and reduced. These atoms will have had a change in their oxidation number. Draw a line to connect them. If they’re not already balanced, add coefficients to balance them. State the total number of electrons lost & gained in each case

Step 3): balance electrons to make sure the number lost = the number gained by multiplying by the appropriate factor

Step 4): Balance the redox participants by putting the multiplying factors from step 3) into the main equation

Step 5): Balance all other atoms by inspection

Step 6): Rewrite the completed balanced equation without all the extra numbers
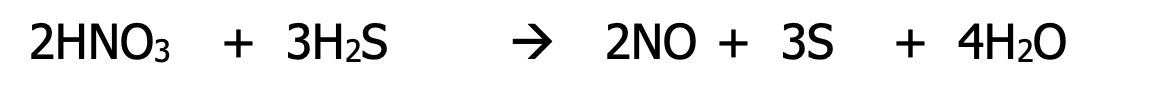
Sheet 4 (p. 27)
Now we will be given a skeleton equation to balance
In a skeleton equation, only the oxidized and reduced species are shown. You have to add the necessary conditions (acid or base) but the question will always specify which
Example: balance the following skeleton equation using the oxidation number method under acidic conditions
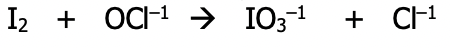
Step 1): Follow steps above 1-4
Step 1): assign oxidation numbers to all atoms in the equation
Step 2): identify the atoms which have been oxidized and reduced. These atoms will have had a change in their oxidation number. Draw a line to connect them. If they’re not already balanced, add coefficients to balance them. State the total number of electrons lost & gained in each case
Step 3): balance electrons to make sure the number lost = the number gained by multiplying by the appropriate factor
Step 4): Balance the redox participants by putting the multiplying factors from step 3) into the main equation

Step 2): At the inspection step, balance oxygen by adding water and hydrogen by adding H¹⁺. Look at both sides of the equation for atoms of hydrogen
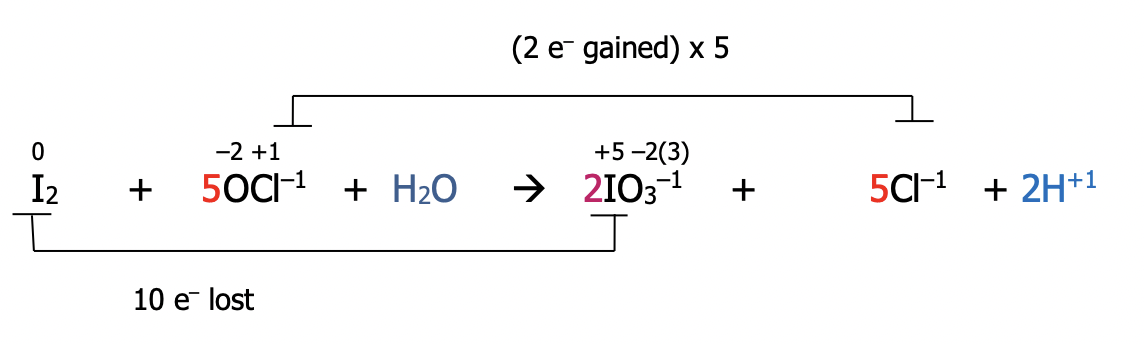
Example: Balance the following redox reaction using the oxidation number method under basic conditions

Step 1): Follow all previous steps, as if the reaction occurs in acid
Step 1): Follow steps 1-4
Step 1): assign oxidation numbers to all atoms in the equation
Step 2): identify the atoms which have been oxidized and reduced. These atoms will have had a change in their oxidation number. Draw a line to connect them. If they’re not already balanced, add coefficients to balance them. State the total number of electrons lost & gained in each case
Step 3): balance electrons to make sure the number lost = the number gained by multiplying by the appropriate factor
Step 4): Balance the redox participants by putting the multiplying factors from step 3) into the main equation
Step 2): At the inspection step, balance oxygen by adding water and hydrogen by adding H¹⁺. Look at both sides of the equation for atoms of hydrogen

Step 2): Now add hydroxide ions to each side equal to the number of hydrogen ions. Adding the same value to both sides of an equation maintains the equality
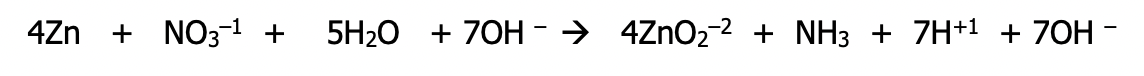
Step 3): Simplify by recognizing that 1 H⁺
Sheet 5 (p. 33)
The half-cell method of balancing redox equations offers an alternate way to achieve balance
Half-cell steps vs. oxidation method steps
Difference in order of operation
The other atoms are balanced first and the electrons are balanced last in the half-cell method
Balancing reactions in acidic solution
Example: Balance using the half-cell method in acidic medium
Step 1): Divide the equation into 2 possible half reactions - it isn’t needed to know which half is oxidation and which half is reduction at this point
 Step 2): Balance each half reaction separately (balance all elements besides H and O first)
Step 2): Balance each half reaction separately (balance all elements besides H and O first)
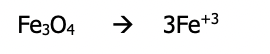 Step 3): If needed, balance the O atoms by adding H₂O to the side needing more O
Step 3): If needed, balance the O atoms by adding H₂O to the side needing more O
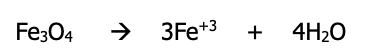 Step 4): Balance the H atoms by adding H⁺ to the side that needs it
Step 4): Balance the H atoms by adding H⁺ to the side that needs it
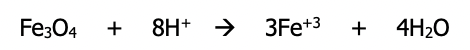 Step 5): Balance for charge (the total charge on the left side must be ===== to total charge on the right side) by adding electrons to the side with greater overall positive charge
Step 5): Balance for charge (the total charge on the left side must be ===== to total charge on the right side) by adding electrons to the side with greater overall positive charge
 This equation is the actual oxidation half reaction where electrons are released
This equation is the actual oxidation half reaction where electrons are released
Step 6): Repeat the above steps to balance the other half reaction. Since the one above turned out to be an oxidation, the next one should be a reduction (electrons required). This equation will be the actual reduction half reaction
 Step 7): Adjust the coefficients of the two balanced half-reactions such that the number of electrons lost is = to the number of electrons gained
Step 7): Adjust the coefficients of the two balanced half-reactions such that the number of electrons lost is = to the number of electrons gained
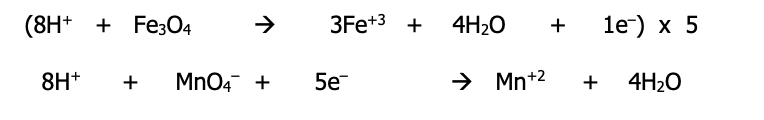 Step 8): Add the 2 reactions to obtain the balanced equation for the redox reaction. Simplify H⁺ and H₂O if needed
Step 8): Add the 2 reactions to obtain the balanced equation for the redox reaction. Simplify H⁺ and H₂O if needed
Since the number of electrons lost and gained are now equal, they should cancel out
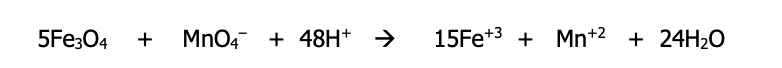
Sheet 6 (p. 40)
Using half-cell method for basic solution
As with the oxidation method, in basic solution you follow the exact same method for acid and then add OH⁻ to both sides to adjust to base
Example: Balance using the half-cell method in basic medium
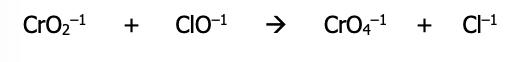 Step 1): Balance each half reaction as if it occurs in acidic solution using the steps described earlier
Step 1): Balance each half reaction as if it occurs in acidic solution using the steps described earlier
Step 1): Divide the equation into 2 possible half reactions - it isn’t needed to know which half is oxidation and which half is reduction at this point
Step 2): Balance each half reaction separately (balance all elements besides H and O first)
Step 3): If needed, balance the O atoms by adding H₂O to the side needing more O
Step 4): Balance the H atoms by adding H⁺ to the side that needs it
Step 5): Balance for charge (the total charge on the left side must be = to total charge on the right side) by adding electrons to the side with greater overall positive charge
Step 6): Repeat the above steps to balance the other half reaction. Since the one above turned out to be an oxidation, the next one should be a reduction (electrons required). This equation will be the actual reduction half reaction
Step 7): Adjust the coefficients of the two balanced half-reactions such that the number of electrons lost is = to the number of electrons gained
Step 8): Add the 2 reactions to obtain the balanced equation for the redox reaction. Simplify H⁺ and H₂O if needed
Since the number of electrons lost and gained are now equal, they should cancel out
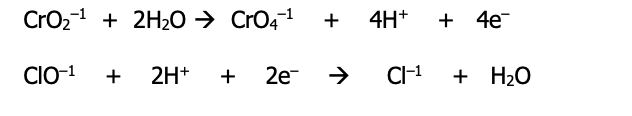 Step 2): Neutralize the H⁺ ions by adding an equal number of OH⁻ ions to both sides of the equation (H⁺ + OH⁻ = H₂O) & simplify
Step 2): Neutralize the H⁺ ions by adding an equal number of OH⁻ ions to both sides of the equation (H⁺ + OH⁻ = H₂O) & simplify
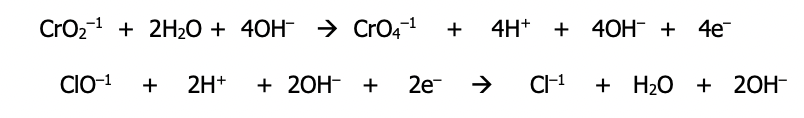 Step 3): Remember that H⁺ + OH⁻ = H₂O & simplify
Step 3): Remember that H⁺ + OH⁻ = H₂O & simplify
 Step 4): Adjust the coefficients of the two balanced half reactions such that the number of electrons lost equals the number of electrons gained. Add the two reactions and simplify further if needed
Step 4): Adjust the coefficients of the two balanced half reactions such that the number of electrons lost equals the number of electrons gained. Add the two reactions and simplify further if needed

Sheet 7 (p. 47)
Galvanic cells (aka Voltaic or Electrochemical. cells) harness the potential energy of a redox reaction and use it to do useful work (e.g. a 1.5 V battery is a Galvanic cell)
In all redox reactions, the reducing agent (which loses electrons) passes electrons to the oxidizing agent (which gains electrons)
A single displacement reaction is a simple example of a redox reaction that can be used in an electrochemical cell
e.g.
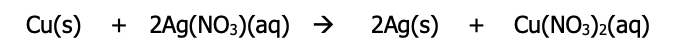
Anytime a single displacement reaction is done, the metal higher on the activity series will lose electrons (and be oxidized) and the metal below it will thus gain electrons (and be reduced)
Activity series: 
If the 2 half reactions are physically separated and connected by a conductor (a wire), then the electrons can only be transferred through the wire from one element to another
Diagram of a Galvanic/electrochemical cell:

All of the substances involved in the reaction must be present in the electrochemical cell assembly however the oxidation & reduction reactions happen in their own compartment: referred to as half-cells
In the diagram’s case above, each half cell contains a beaker of an ionic solution, where the cation is the metal ion involved in the reaction and a strip of the same metal element partially immersed in the solution. This strip of metal is called an electrode
For this example, there is a piece of cooper metal immersed in a copper (II) nitrate solution (usually 1.0 M solutions are used) and a piece of silver metal immersed in a silver nitrate solution. Nitrate is a good anion to pair with each cation because all cations are soluble with it
The cathode: the electrode where reduction takes place
The anode: the electrode where oxidation takes place
The anode is where the electrons originate. Because of this, the anode is more negative relative to the cathode & so the anode is labelled as the negative electrode and the cathode as the positive electrode
This corresponds to the negative and positive terminals of a cell or battery
The redox used in the above example is a single displacement but any reaction can form the basis of an electrochemical cell
In many redox reactions (but not all) the electrodes are involved in the reaction. The electrodes are only involved if the reaction includes solid metal atoms that become metal cations or vice versa
When metal atoms of the anode loses electrons to become cations which enter/dissolve into the solution, the solution becomes positively charged and the mass of the anode decreases. It decreases because the neutral atoms have become ions and have left the electrode for the solution. This is illustrated by the following net (the spectator anion nitrate has been removed) oxidation of half reaction

At the cathode, ions in the solution pick up the incoming electrons & are reduced to become neutral metal atoms. They deposit on the electrode so its mass increases. The solution begins to become negatively charged because the cations are consumed from the solution (the anions that are in the solution will now be greater in number)
This is illustrated by the following net (the spectator anion has been removed) reduction half reaction
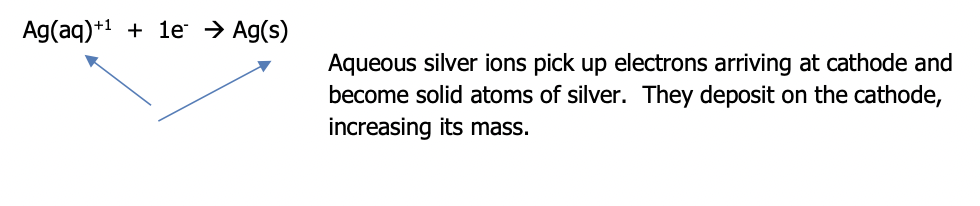
The net redox reaction is the sum of the two half reactions (note that the silver half reaction will need to be multiplied by 2 to get the electrons lost to those gained)
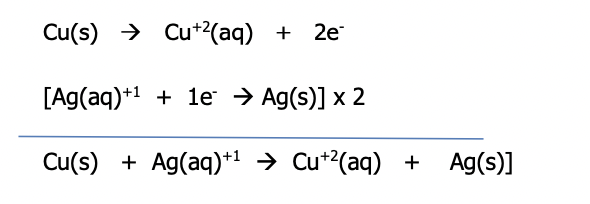 Reconsider the diagram of the Galvanic cell
Reconsider the diagram of the Galvanic cell
Salt Bridges
There is a secondary connection between the half-cells called a salt bridge
Salt bridge: needed to connect the two half cells to allow ions to migrate back and forth between the half cell solutions.
Recall that the solution containing the anode becomes positively charged over time as metal atoms turn into cations and enter the solutions whereas the solution containing the cathode becomes negatively charged as anions will be in greater number since cations are depleted by the reaction
This building charge in each beaker will stop the flow of electrons through the wire from the anode to the cathode. e.g.) Since the copper ion concentration builds, these cations attract the electrons back faster than they can travel through the wire to the other side to be picked up by silver ions & so the current flow stops
To keep the driving force on, the electrons to go from the anode to the cathode, the electrical neutrality of each solution must be maintained
It can be as simple as a piece of filter paper soaked in soluble salt solution. NaNO₃ and KNO₃ are usually good choices for they tend not to interfere with the reaction & are soluble
Anions always flow towards the anode
The solution is becoming positive so it will attract anions to achieve neutrality
Cations always flow towards the cathode
The solution is becoming negative so it will attract cations to achieve neutrality
Remember that the sign assigned to the anode is negative (-) which is different than the charge building up in the solution, which is positive (+) –– the same is true for the cathode
In a Galvanic cell, electrons always flow from the anode to the cathode through the wire, anions flow to the anode half cell through the salt bridge and cations flow to the cathode half cell through the salt bridge
Inert Electrodes
If a half reaction does not include a solid metal and either the oxidation or reduction half reactions (or both) happen within the solution only or involve gases a metal electrode is still needed to allow the electrons to travel from one hand cell to the other
Platinum (a very inert metal found at the bottom of the activity series) is usually the ideal choice as the electrode since it will not interfere with ongoing reactions
The platinum electrode will act as a conductor to allow the electrons released from oxidation to trace to the cathode (which could possibly be another platinum electrode if the reduction occurs in solution or involves gases)
Graphite is an allotrope of carbon and can also be used as an inert electrode
Cell Line Notation
Cell line notation is a special notation used to represent electrochemical cells that indicates the redox reaction occurring
Rules:
The anode half cell is described first followed by the cathode half cell
Within a given half cell, the reactants are specified first and the products last – the description of the oxidation reaction is first and the reduction reaction is last – when you read it, your eyes move in the direction of electron flow – spectator ions aren’t usually included
A single vertical line is drawn between 2 chemical species that are in different phases but in physical contact with each other (e.g. solid electrode | aqueous solution)
A double vertical line (||) represents a salt bridge or porous membrane separating the individual half cells
The phase of each chemical (i.e. s, l, g, aq) is shown in brackets – if the electrolytes in the cells aren’t at STP it must be included in brackets with the phase notation, if not noted then the electrolytes are assumed to be at standard conditions

Sheet 8 (p. 54)
Standard Electrode Potentials
In a galvanic cell, electrons are produced at the anode (oxidation) and picked up at the cathode (reduction)
Means that the anode will will be more negative than the cathode
Anode is often labelled negative & the cathode is labelled + but the cathode is actually just less negative
Voltmeter: compares the difference in this charge build up between the electrodes
The greater the difference, the greater the voltage reading (aka potential difference or electromotive force)
It essentially measures the “electric pressure” that exists between the anode & cathode or the propensity for the electrons to move from the anode to the cathode
When the substance being oxidized has a very high tendency to lose electrons (strong reducing agent) and the one being reduced has a very high tendency to gain (strong oxidizing agent) – there will be a greater potential difference between them and thee combination will result in a higher voltage reading than if they have more similar oxidation/reduction potentials
A waterfall is a good analogy – the greater the drop, the greater the gravitational potential difference
The voltage or potential difference of an electrochemical cell under standard conditions has the symbol Eºcell
Impossible to measure the potential or voltage of a half cell alone – to get around this, there is a standard that everything is compared to, this is called the hydrogen half cell
This reference half cell consists of a Pt electrode in a solution containing 1 M H⁺ into which H₂ gas is added at a constant pressure of 1 atm and is assigned a potential of 0 V
The reaction is:
H⁺ (aq) + 2 e⁻ → H₂ (g) Eºr = 0 V
The cell potential measured for a given half-cell connected to hydrogen half-cell is reported as the standard half-cell potential of the substance since the hydrogen value is assigned 0 V
By convention, standard reduction potentials (symbolized by Eºr) are reported rather than both reduction and oxidation potentials - these potentials are a measure of the tendency of a given half-cell to gain electrons from the hydrogen half-cell


Sheet 9 (p. 58)
Spontaneity rule: a redox reaction will be spontaneous if the oxidizing agent is above the reducing agent on a table of reduction potentials and the overall cell potential will be positive
The overall cell potential (Eºcell) can be obtained by adding the potentials pf the reduction (Eºr) and of the oxidation had reactions (Eºox)
Reverse the sign of the potential when flipping the reduction equation to get the oxidation equation

If the half reaction is multiplied by a factored to make the number of electrons lost equal that gained – do not multiply the potential as it isn’t affected by the number of electrons transferred (these are separate ideas and quantities)
Example: use a redox table to determine if a spontaneous redox reaction can occur. If so, find the most spontaneous redox reaction that will occur, write the equations for all relevant reactions and the net reaction and calculate the cell potential
Tin (II) bromide is poured into a solution of acidified sodium dichromate
Step 1: list all entities present
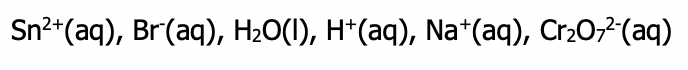
- Aqueous ionic compounds must be split into their ions
- Covalent compounds remain intact even when aqueous unless they are acids which also form ions
- Do not forget to list water if conditions are aqueous
- Do not forget to list H⁺ if conditions are acidic or OH⁻ if conditions are basic
- Bromine is the diatomic element, but bromide is the ion and the same applies to the other halogens
Step 2: classify entities as either oxidizing agents (left side of the equation in a redox table) or reducing agents (right side of the equation in redox table)
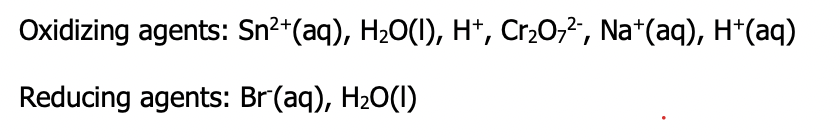
- Go down the table and highlight/circle each entity present in solution
- Circle only the exact entity to have no matter which side it appears on

- Only circle a given substance, if all the reactants are present in the mixture, otherwise the reaction can not occur and so is not a possibility (products irrelevant).
-- In this example, there are other reactions that contain water, for instance, besides the ones that were selected, but they could not be used because they contained additional reactants that were not present in the original mixture and so could not occur.
- If your substance is on the reactant side, the reaction can only occur in the forward direction and so the substance can potentially be reduced and is an oxidizing agent.
- If your substance appears on the product side, the reaction can only occur in the reverse direction, and so the substance can only be oxidized and is a reducing agent.
Step 3: identify the strongest oxidizing agent (closest to top left) and strongest reducing agent (closest to bottom right)
-- consider all substance you circle on the left side of each reaction and choose the one closes to the top (highest one)- it is most likely to be reduced (strongest oxidizing agent)
-- consider all substances you circled on the right side of each reaction, choose the one closest to the bottom (lowest one) - it is most likely to be oxidized (strongest reducing agent)
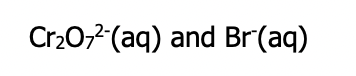
Step 4: Apply spontaneity rule
Since the strongest oxidizing agent is above the strongest reducing agent a spontaneous redox reaction will occur & by picking the strongest oxidizing and strongest reducing agents, this combo will produce the greater potential difference thus being the most spontaneous
- The same substance can appear in more than 1 reaction or be both oxidized & reduced
-- never use a reaction unless you have all reactants included in that reaction in the mixture.
--- If the reaction occurs as a reduction, the reactants are those substances on the left side of the equations in the redox table. If the reaction occurs as an oxidation, the reactants are those substances on the right side of the equations in the redox table. If a reaction involving nitrate ion requires acidic conditions and none are present, for example, that reaction cannot occur, even if the nitrate ion is the strongest OA. Use the next strongest OA, provided all reactants are found in the mixture.

Step 5: write the half-reactions and the net reaction
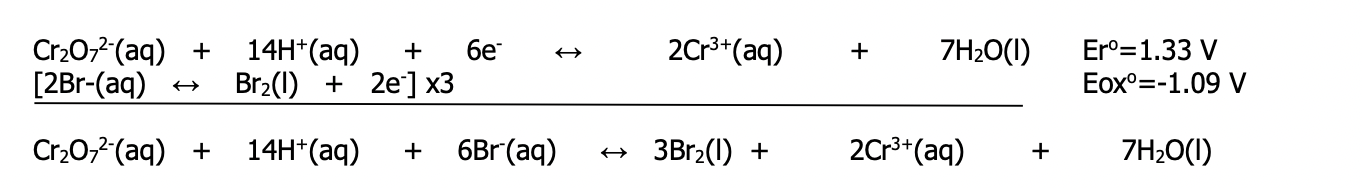
-- Make sure the number of electrons lost = gained by multiplying the entire equation by the appropriate factor
-- Do not multiply reduction potentials (Eºr) or oxidation potentials (Eºox) by this factor
Step 6: Calculate the standard cell potential

-- Use the formula (Ecell = Ecathode - Eanode) without changing any of the signs or change the sign of the reduction potential that actually occurs as an oxidation and add the two values
Do not do both or the answer will be wrong
--- In the above example, the sign was reversed from what was shown in the table for the oxidation reaction and so the values were simply added to obtain the cell potential. If using the formula, do not change the sign and use the formula as the (–) in front of the Eanode will do this for you
 Knowt
Knowt