12-05: Proteins
4 calories per gram of energy → Similar in energy to carbs
Most diverse molecules in living organisms
-- Structural support (muscle)
-- Storage (center of glycogen)
-- Transport (hemoglobin; on RBC which transport oxygen)
-- Signalling (hormones and steroids)
-- Cell response (receptor proteins)
-- Movement (contractile proteins)
-- Defense (antibodies, latching onto antigens and identifying pathogens and things that shouldn’t be there)
-- Catalysis of reactions (enzymes)
-- and more!
The body can’t store protein
Extra protein is converted to energy and/or stored as fat (triglycerides, anything in excess is converted to triglycerides)
Proteins aren’t mostly our main source of energy, but we need their amino acids
Amino Acids
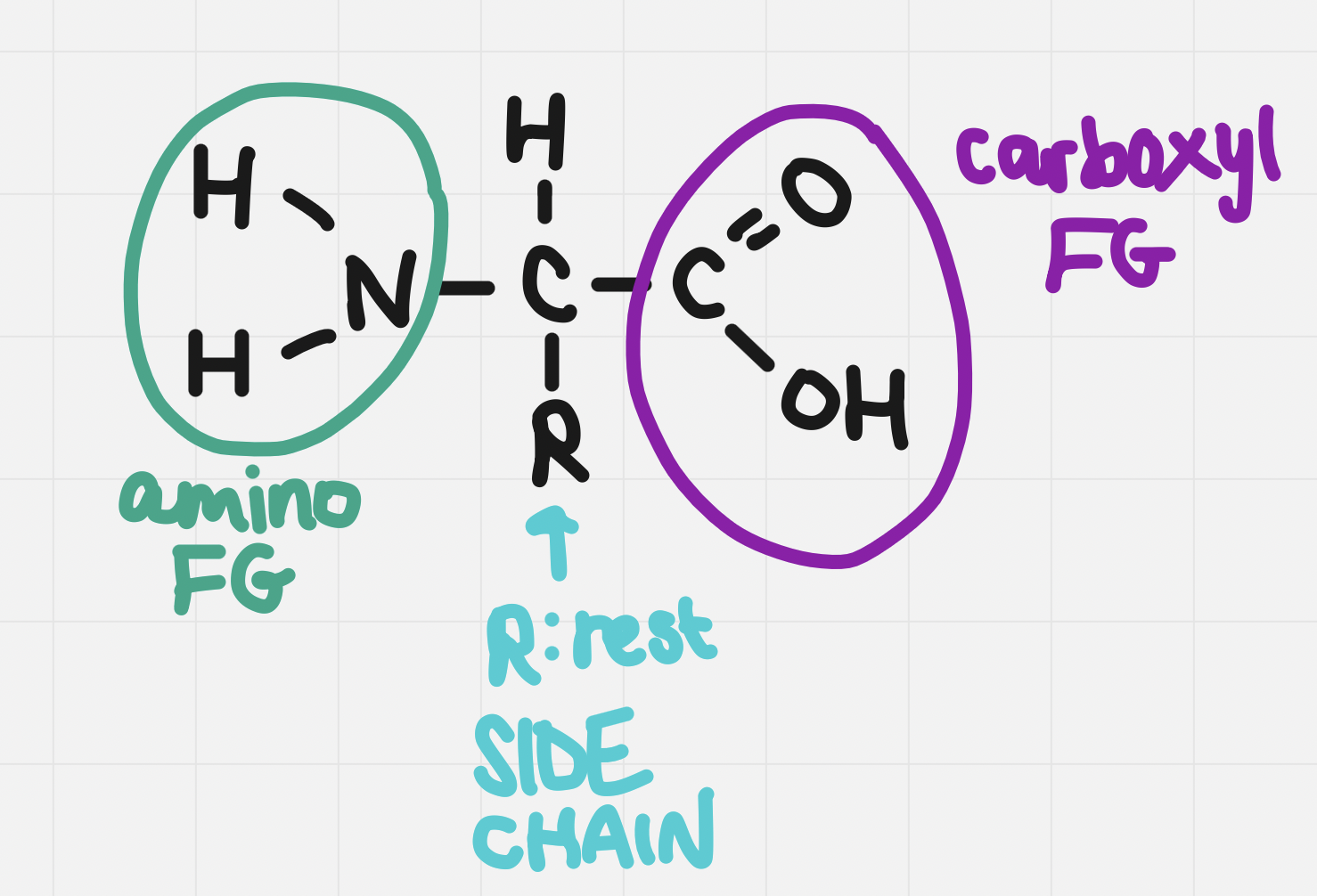
Monomers of proteins (building blocks of proteins)
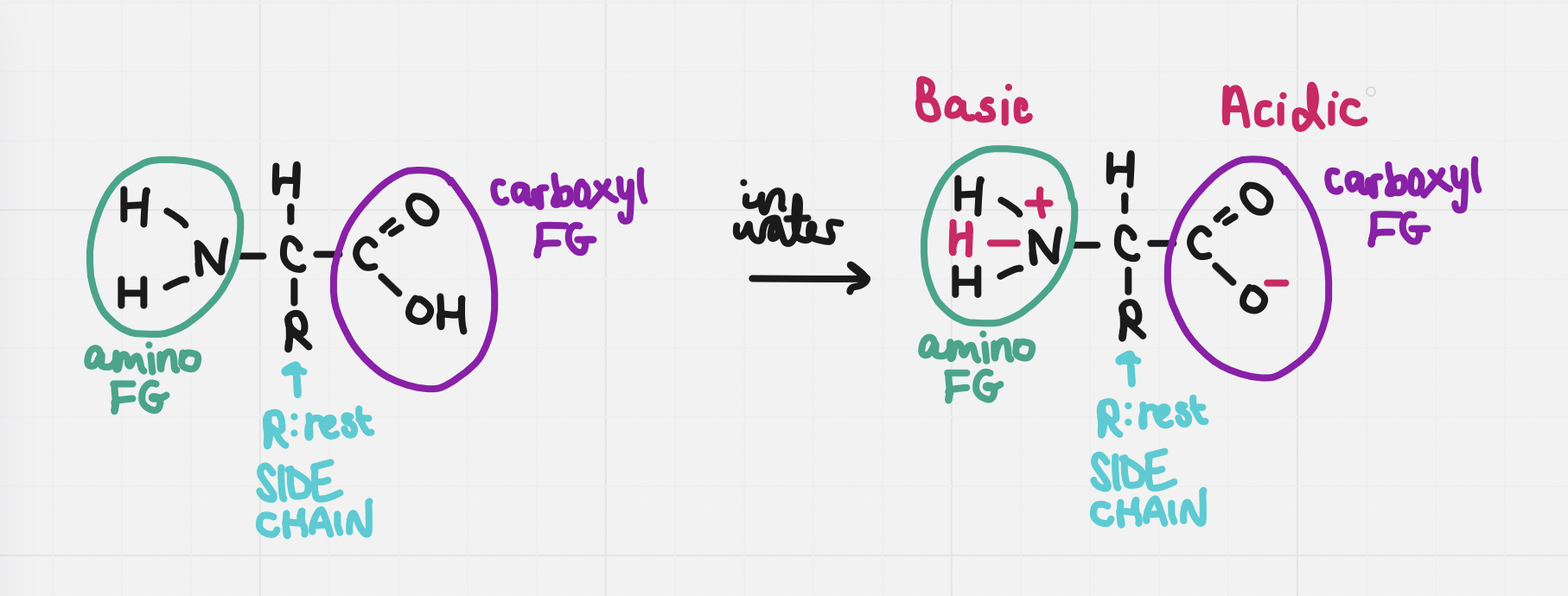
They are amphiprotic - they have both an acidic and basic component
The carboxyl group is acidic and the amino group is basic
There are 20 different side chains and thus 20 different amino acids
9 are essential amino acids which mean that we need them in our food (must be consumed) because our bodies cannot manufacture them ourselves
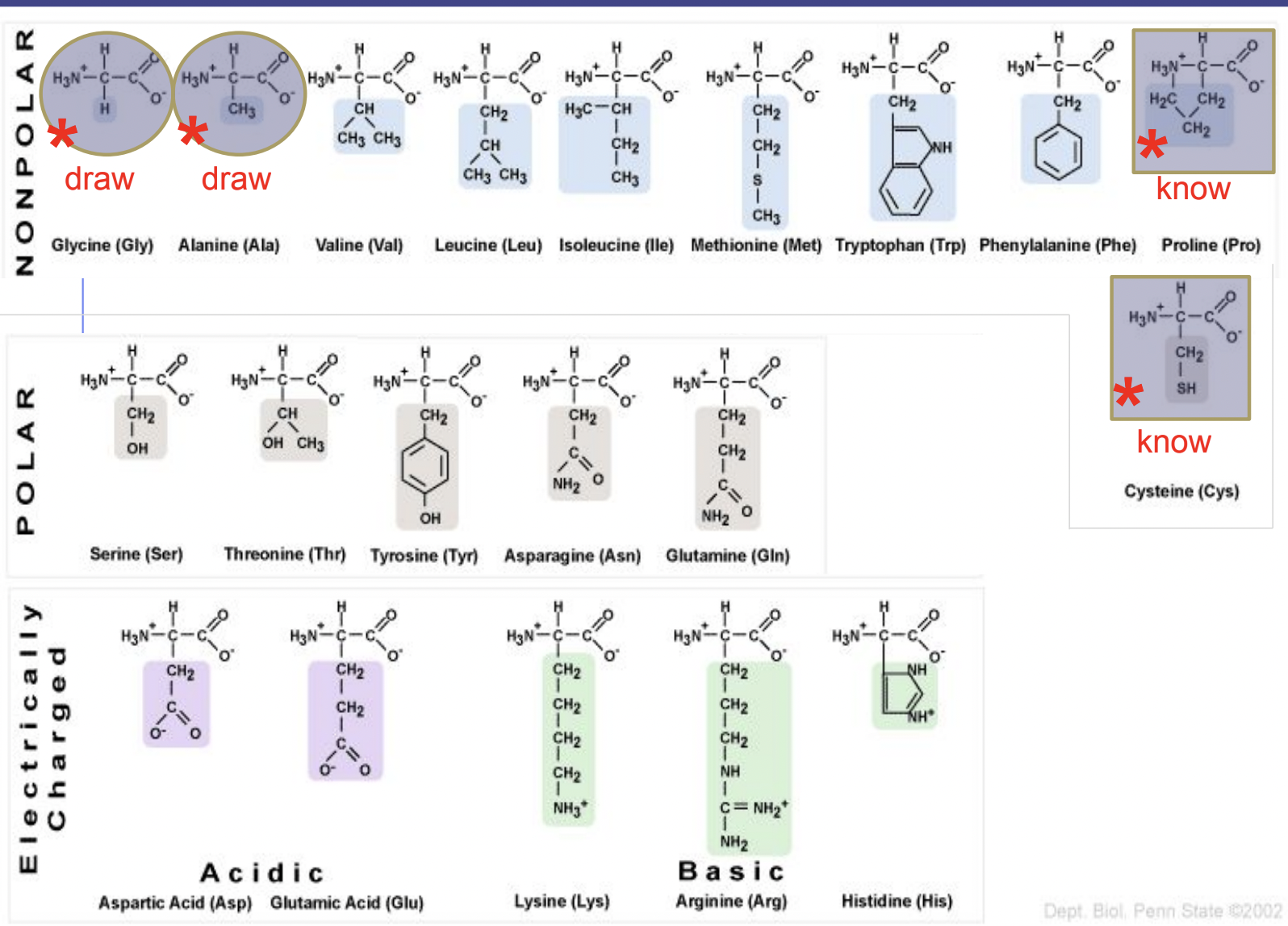
Non polar: are non polar because of ∆EN
C–H and C–C bonds are non polar
Polar: polar because of their functional groups
more hydrophilic
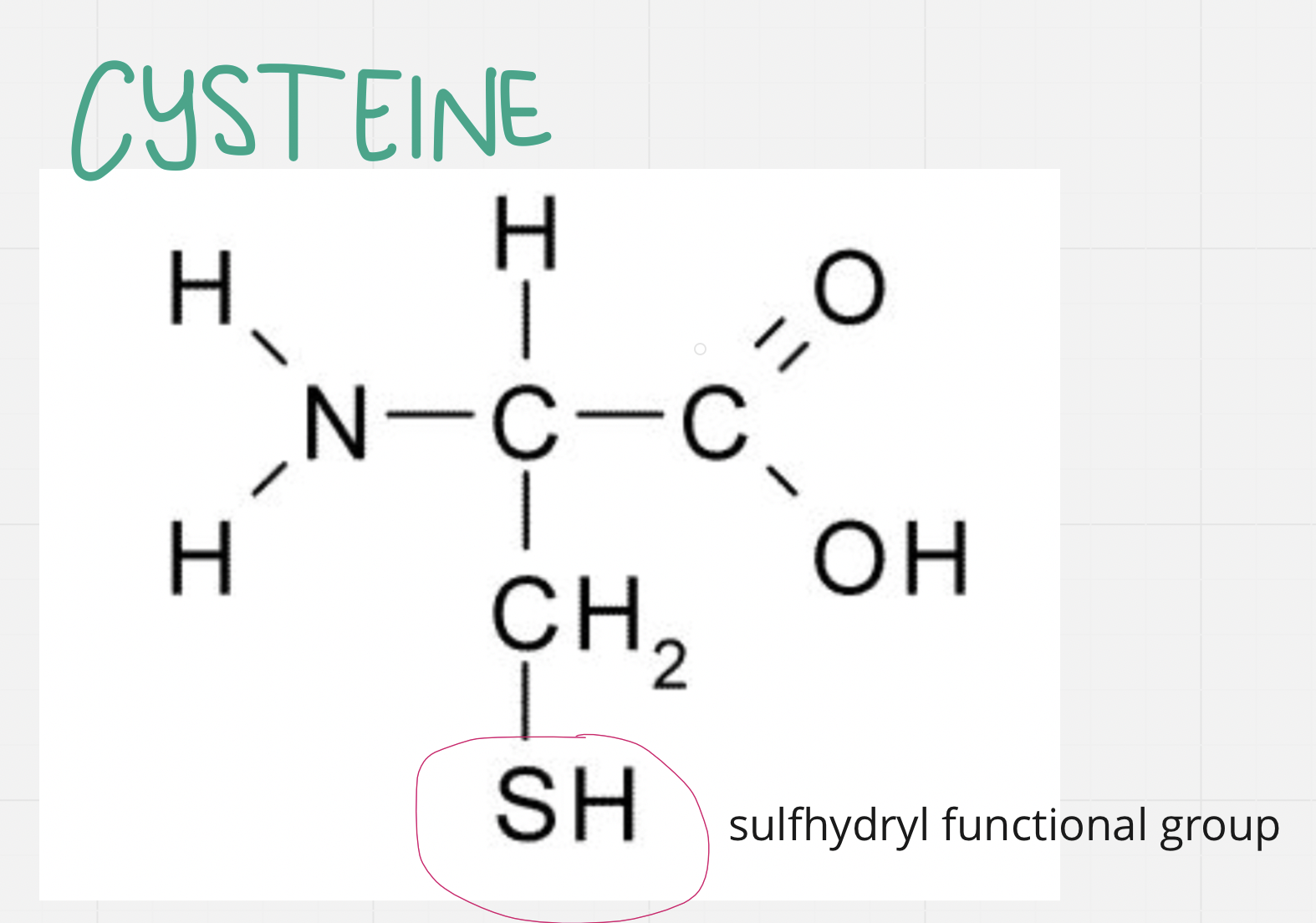
SH on cysteine is a functional group called sulfhydryl – its polarity is debated, it is very weakly polar covalent though some consider it non polar
Creates bonds with other cysteine
Electrically charged: side groups are charged
charged carboxyl = negative charge
charged amino = positive charge
Amino acids to know how to draw
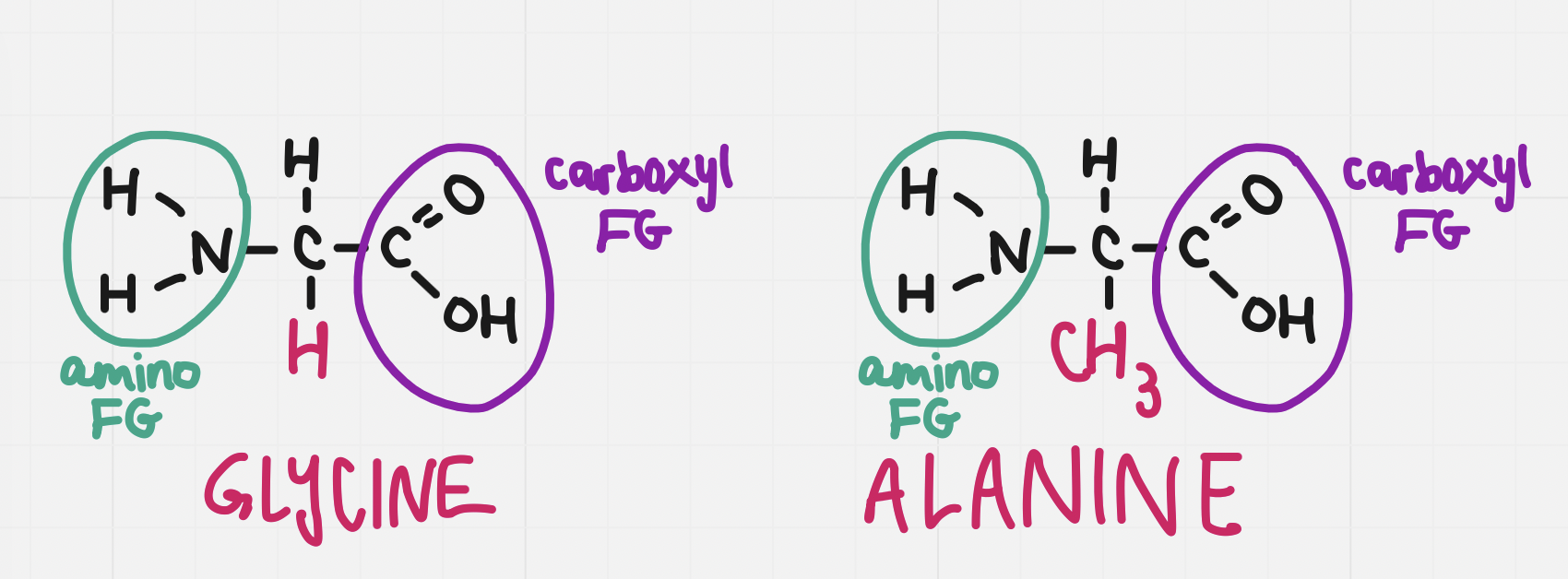
Amino acids to note
Cysteine
Contains S in side chain sulfhydryl FG
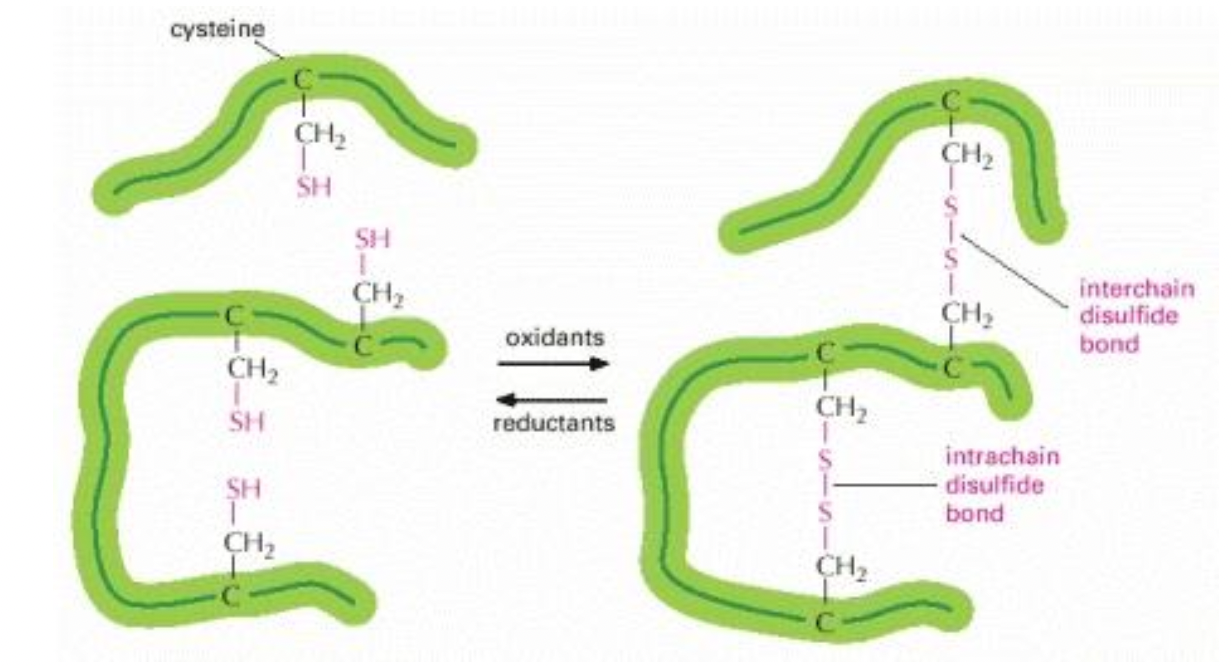
The S forms disulfide bridges (bonds) with other cysteines
Helps hold chains together
2 cysteines close enough will bond
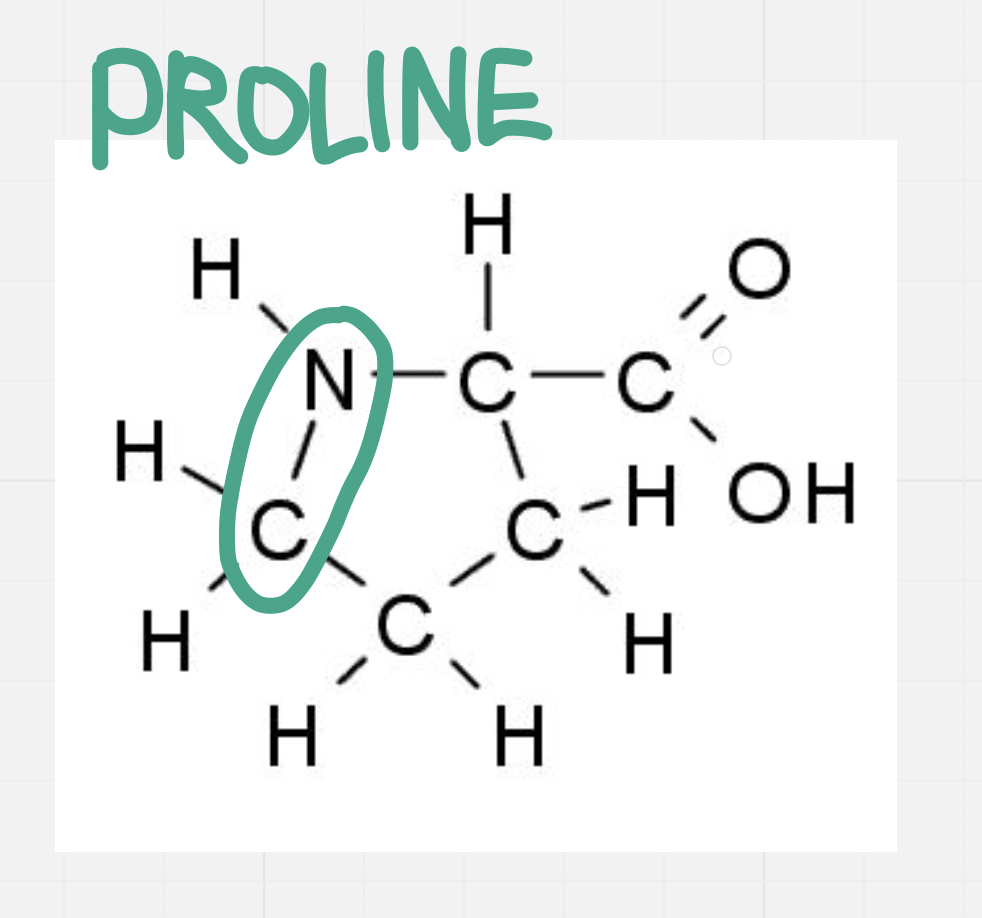
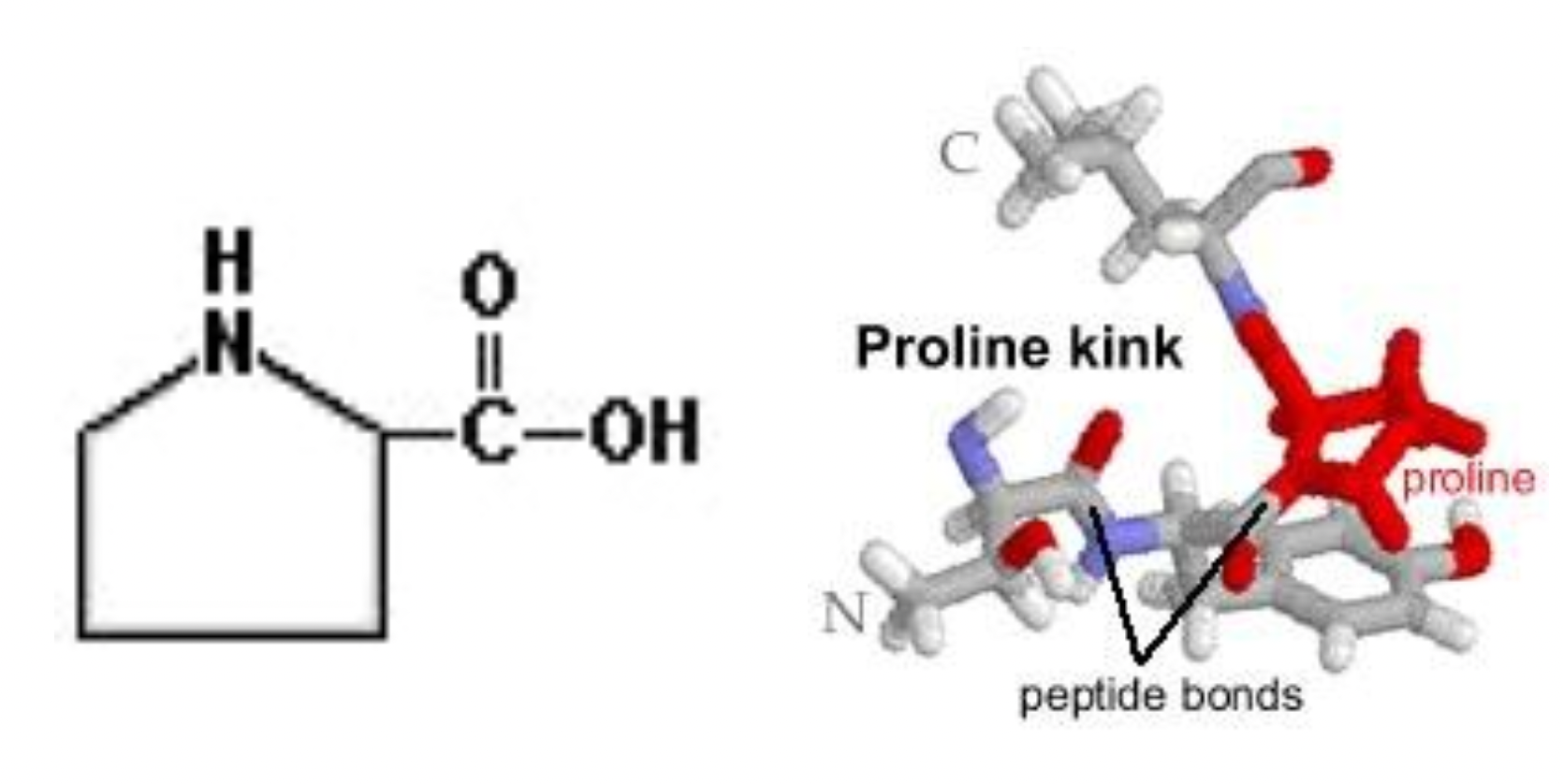
Proline
Side chain forms a ring with itself
Side chain bonds to base/foundation of the molecule
Causes bends in the polypeptide chain
Proline kink = a bend in the strand
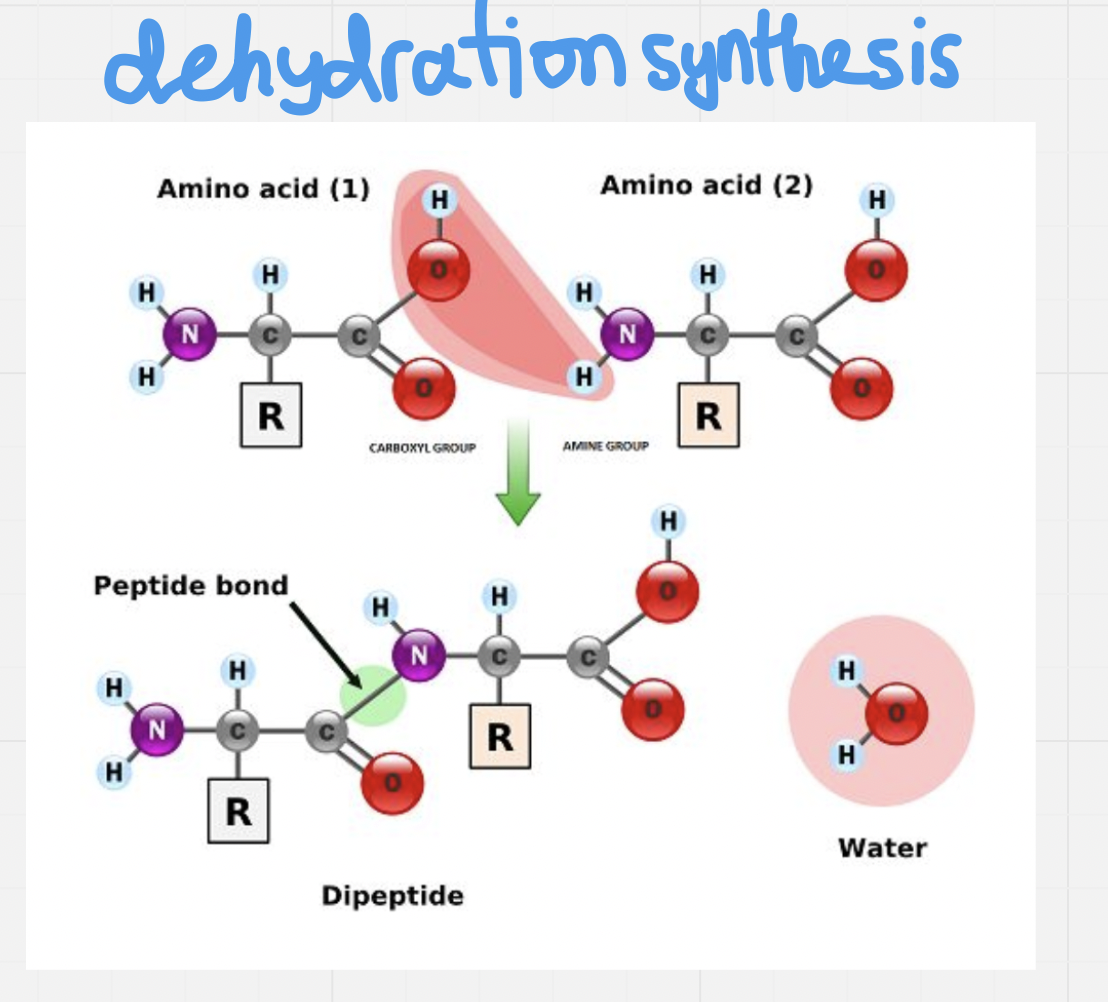
Amino acid formation
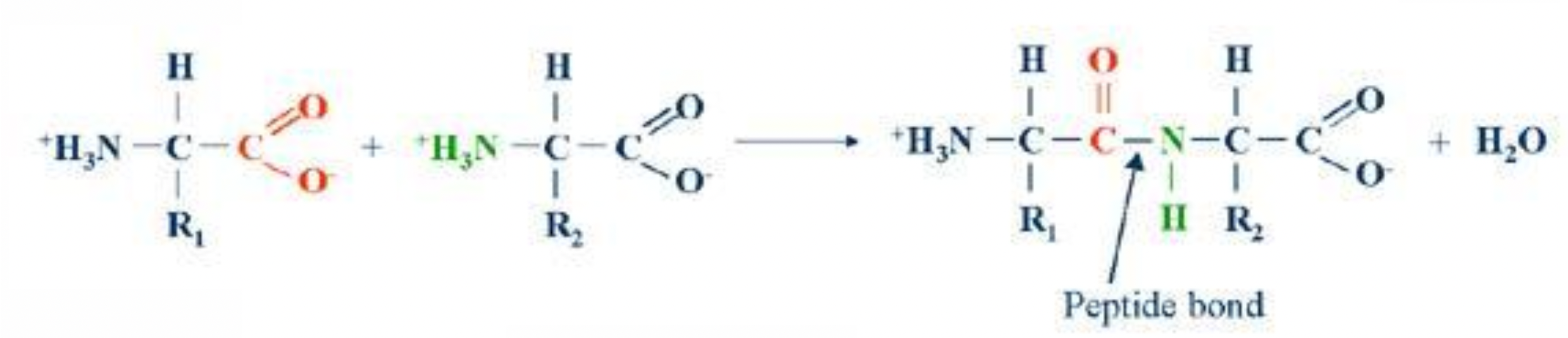
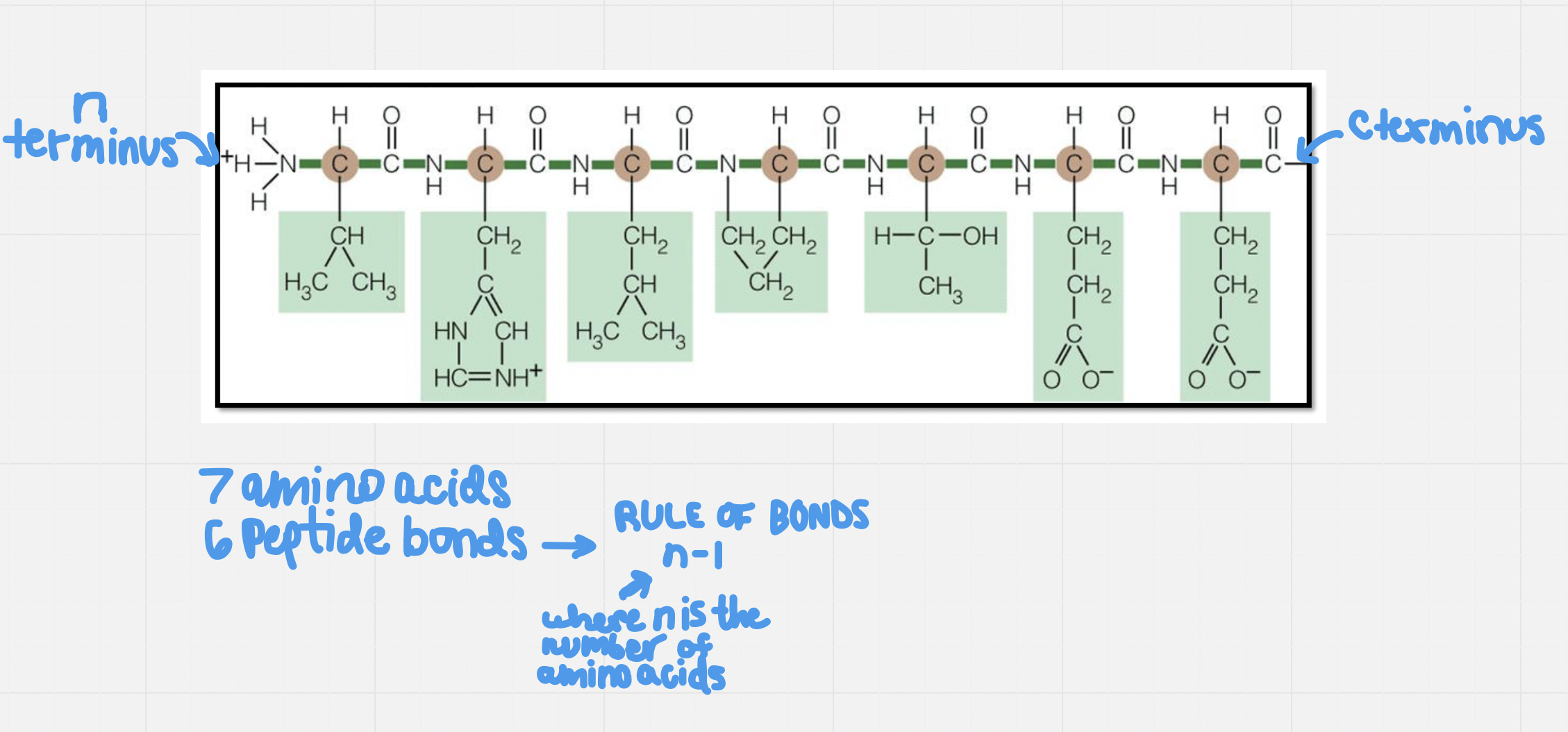
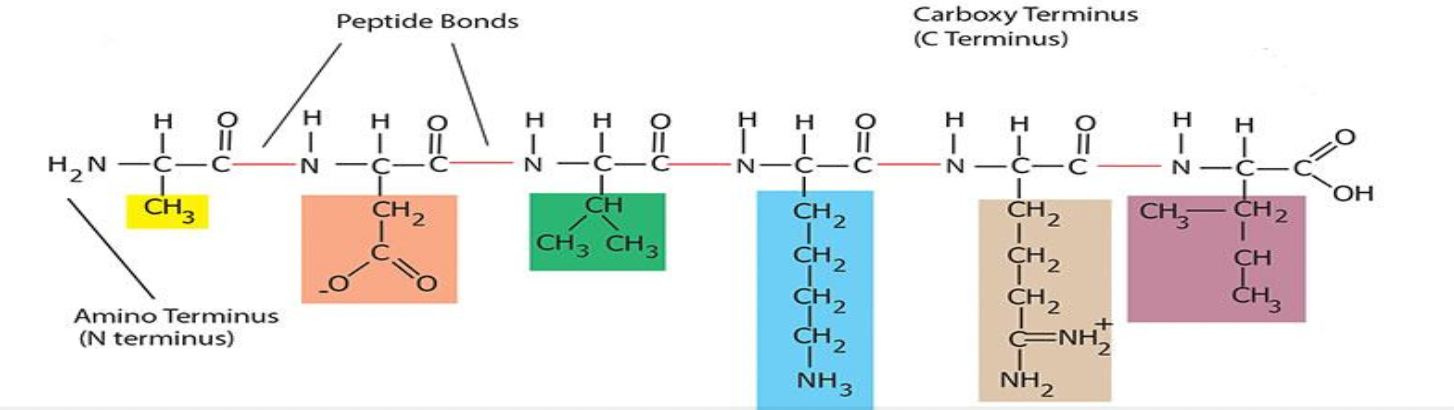
Monomers (amino acids) attach to each other by forming peptide bonds
They form these peptide bonds between the amino group of one amino acid and the carboxyl group of another amino acid
A protein is hundreds of amino acids strung together
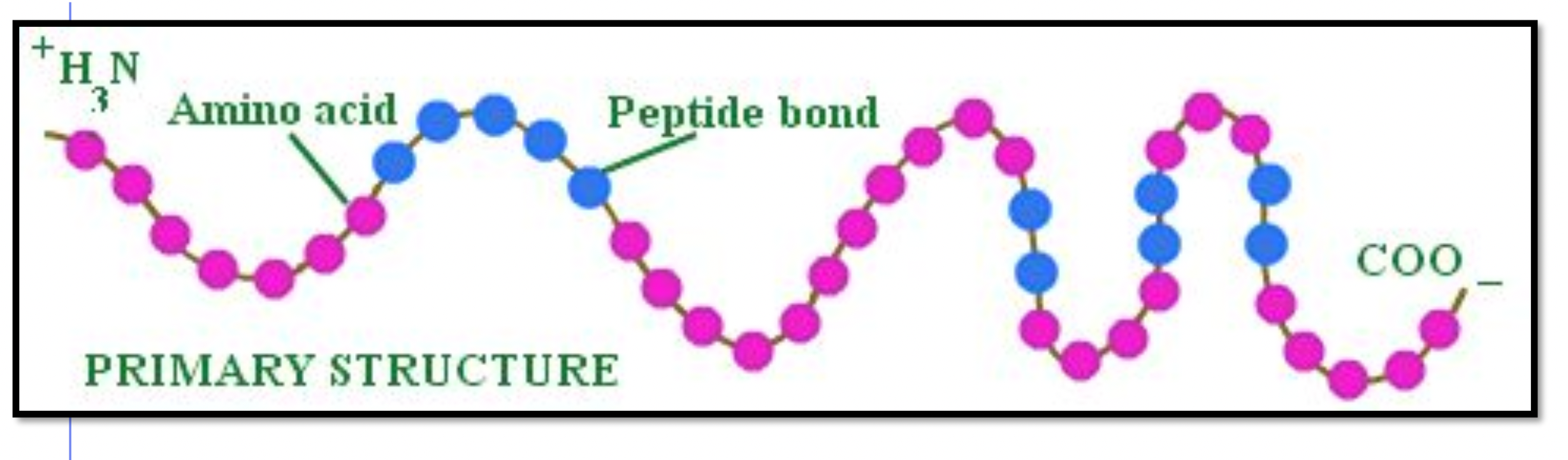
When counting amino acids, count by side chain (every amino acid has one side chain)
Polypeptides
When over 50 amino acids are strung together it forms a polypeptide (many = poly)
The order of amino acids is determined by an organism’s DNA (instruction manual)
Once the polypeptide chain has been formed, it has to be folded into a specific shape in order to properly function
Structure determines function
If there is something wrong with the DNA then an improper protein will be made. Cystic fibrosis is caused by a single protein being wrong
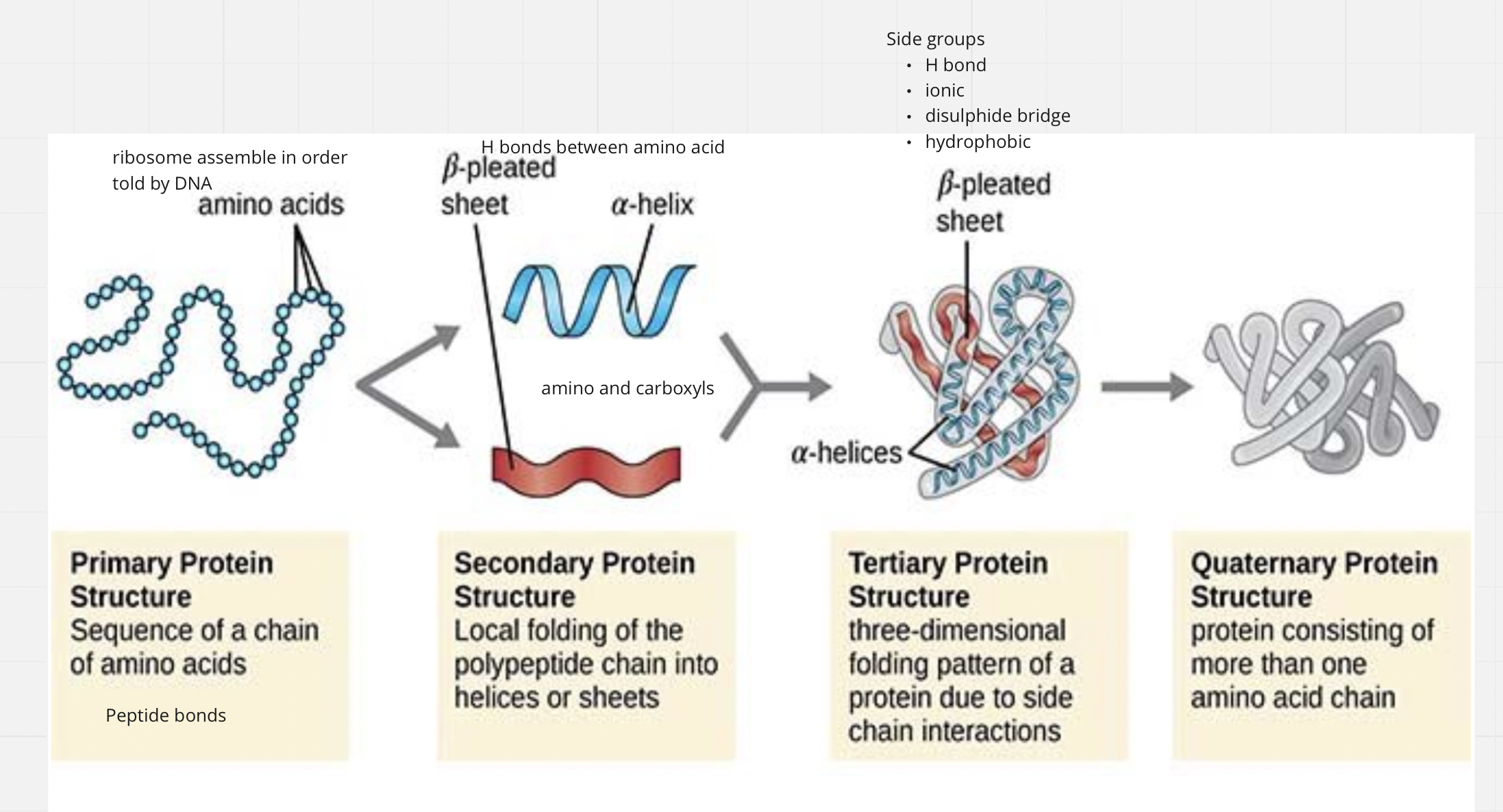
4 levels of folding (only the first 3 are required to reach full protein status)
Primary structure: sequence of amino acids (aka residues)
Secondary structure: coils and folds
Tertiary structure: supercoiling
Quaternary structure: subunit interaction
Primary Structure
Starts at the N (amino) terminus and ends at the C (carboxyl) terminus
Order of amino acids determines how the protein will be folded
A single mistake can cause a huge change in a protein (e.g. sickle cell anemic which causes the RBC to be half moon shaped and causing them to get stuck)
A single mistake in DNA causes a single amino acid in the protein sequence to be different, leading to a serious change in the shape of RBC
Secondary Structure
Coils and folds
Stabilized by Hydrogen bonding between amino and carboxyl groups
α-helices
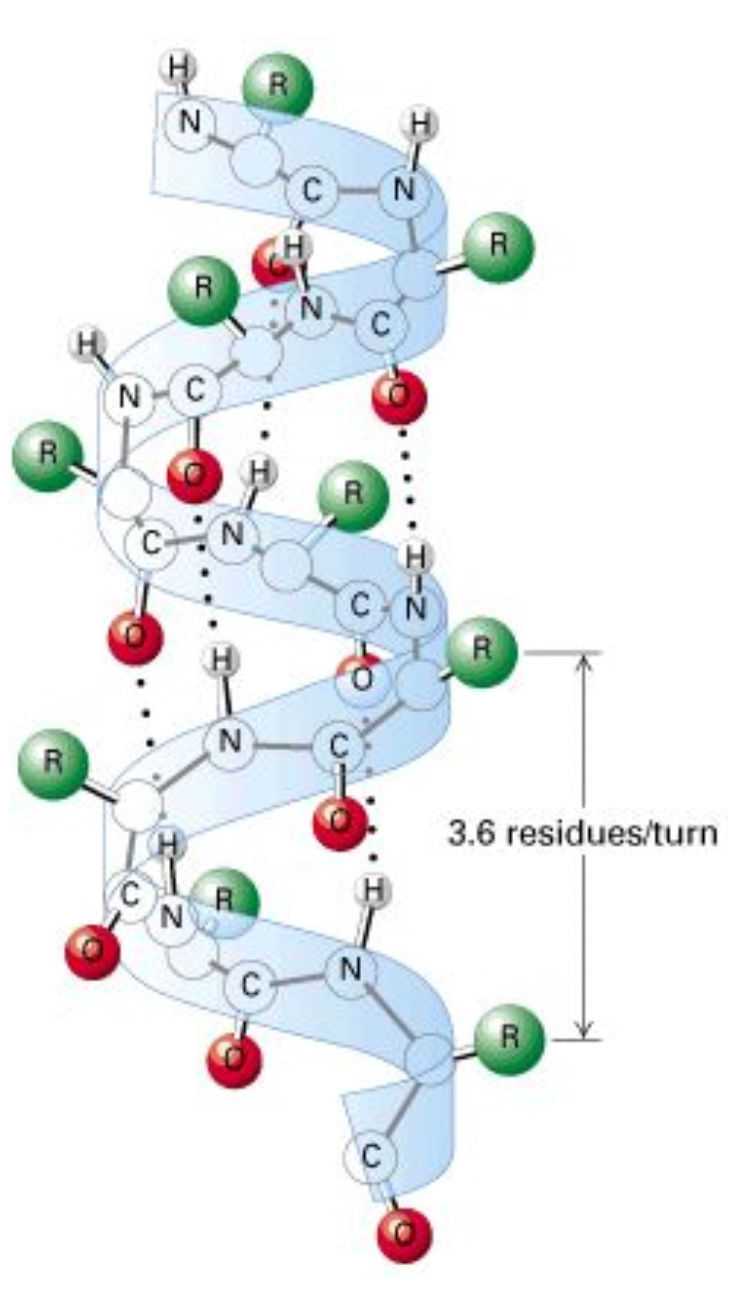
H bonding is forming amino groups to other carboxyl groups
Forms a coil
The carboxyl bonds to amino ~4 carbons away
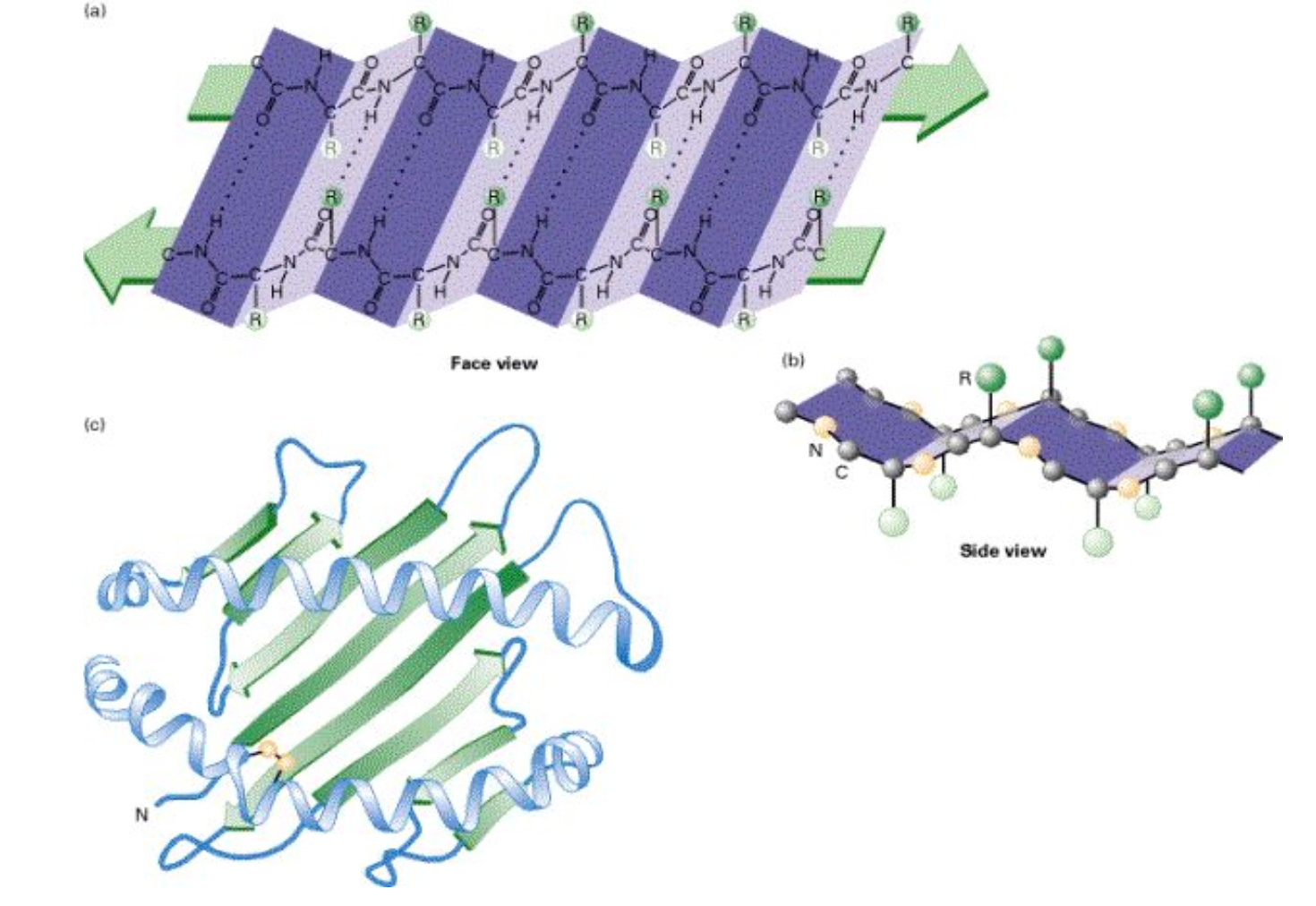
β-pleated sheets
Carboxyl and amino
Instead of the strand staying straight, it will turn a corner and make an S-like curve
Different parts of the protein can coil and sheet (as seen in the bottom image)
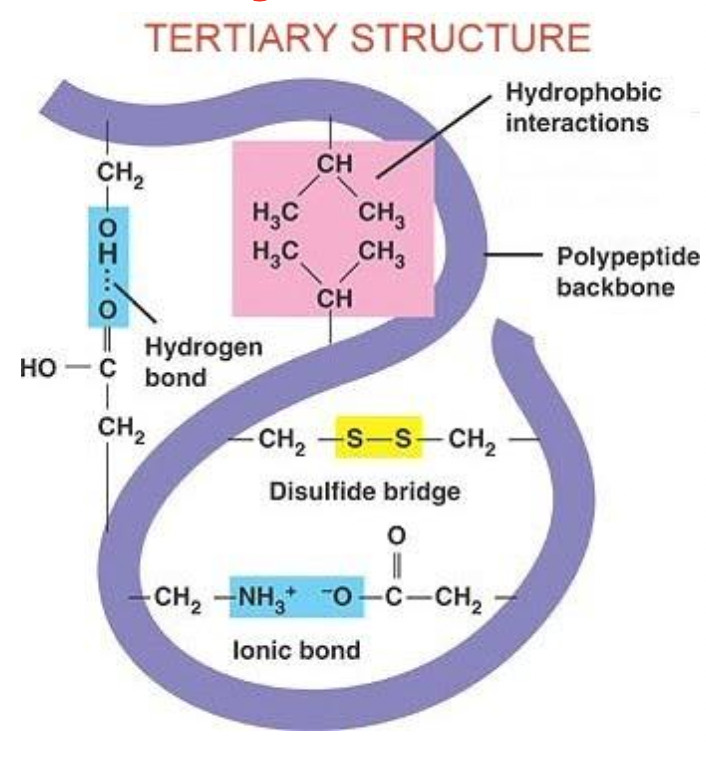
Tertiary Structure
Super coiling occurs at the tertiary level when side groups (R) on the amino acids interact with each other and cause another level of folding
Disulfide bridges (if there is a cysteine amino acid)
Hydrophobic interactions (non polar)
Hydrogen bonding (different in tertiary structure, between side groups (as opposed to the base of an amino acid in secondary))
Ionic bonding (between acidic and basic)
Can be a fully functioning protein at this level
Can be a fibre or globular
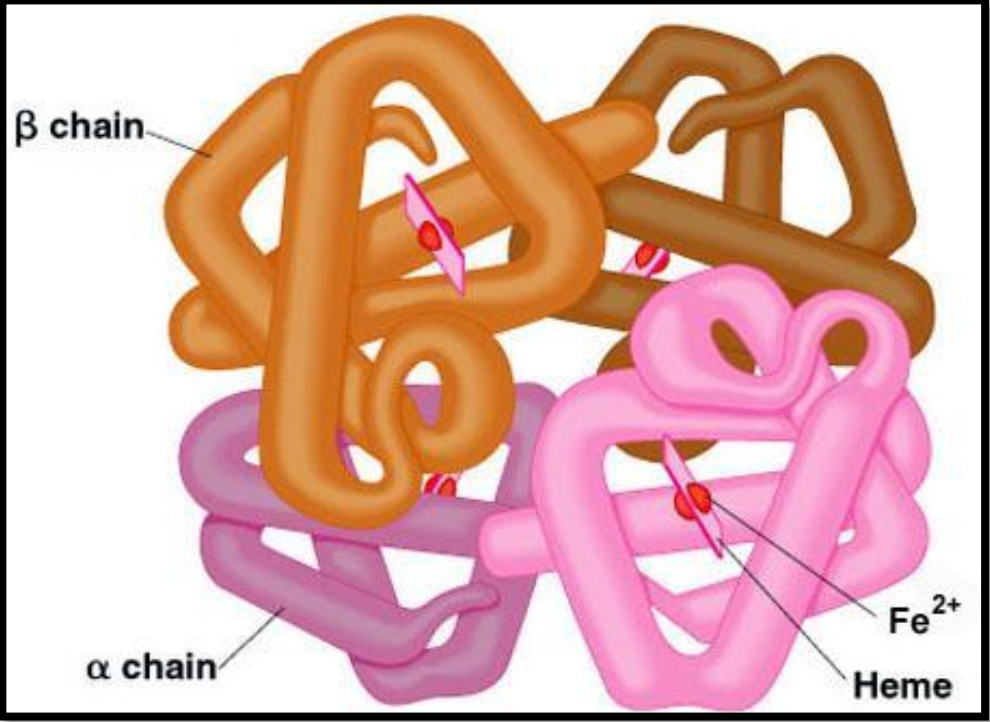
Quaternary Structure
Not all proteins have this -- if a protein is made up of more than 1 polypeptide strand
Some proteins are made of more than one polypeptide (2 or more subunits interacting)
Hemoglobin: has quaternary structure because one hemoglobin is made of 4 polypeptide chains together
Has an accessory molecule – vitamins and minerals: this is called heme and it is made of iron (Fe) → prosthetic group
Some proteins with quaternary structures also have prosthetic groups (non protein components)
Hemoglobin: 4 heme groups which carries 4 oxygen
People with anemia faint, are pale, and are tired. They take iron supplements to attract the oxygen
Summary
Types of proteins
Globular
One or more polypeptide chains that take on a round spherical shape (forms a blob)
E.g. antibodies, enzymes
Hemoglobin are globular
Can be one or many chains
Fibrous
Long strands or sheets of polypeptide chains
E.g. actin and myosin (muscle contractions), silk, collagen (skin), and keratin (hair)
Pile up to form fibrous tissue (doesn’t ball up, more of a linear structure)
Changing of proteins
Proteins can only function in their optimal shape
Their shape can be impacted by their environment
The 3D shape of a protein can be changed by changes in temperature, pH, or ionic concentration
The loss of shape is called denaturation (if it isn’t in its native conformation/optimal shape)
Therefore proteins have optimal ranges for temperature, pH, and ionic concentration within the function’
Can be rendered useless if the shape is too altered and cannot be reversed if the primary structure is altered (if slightly changed it can still be sort of useless)
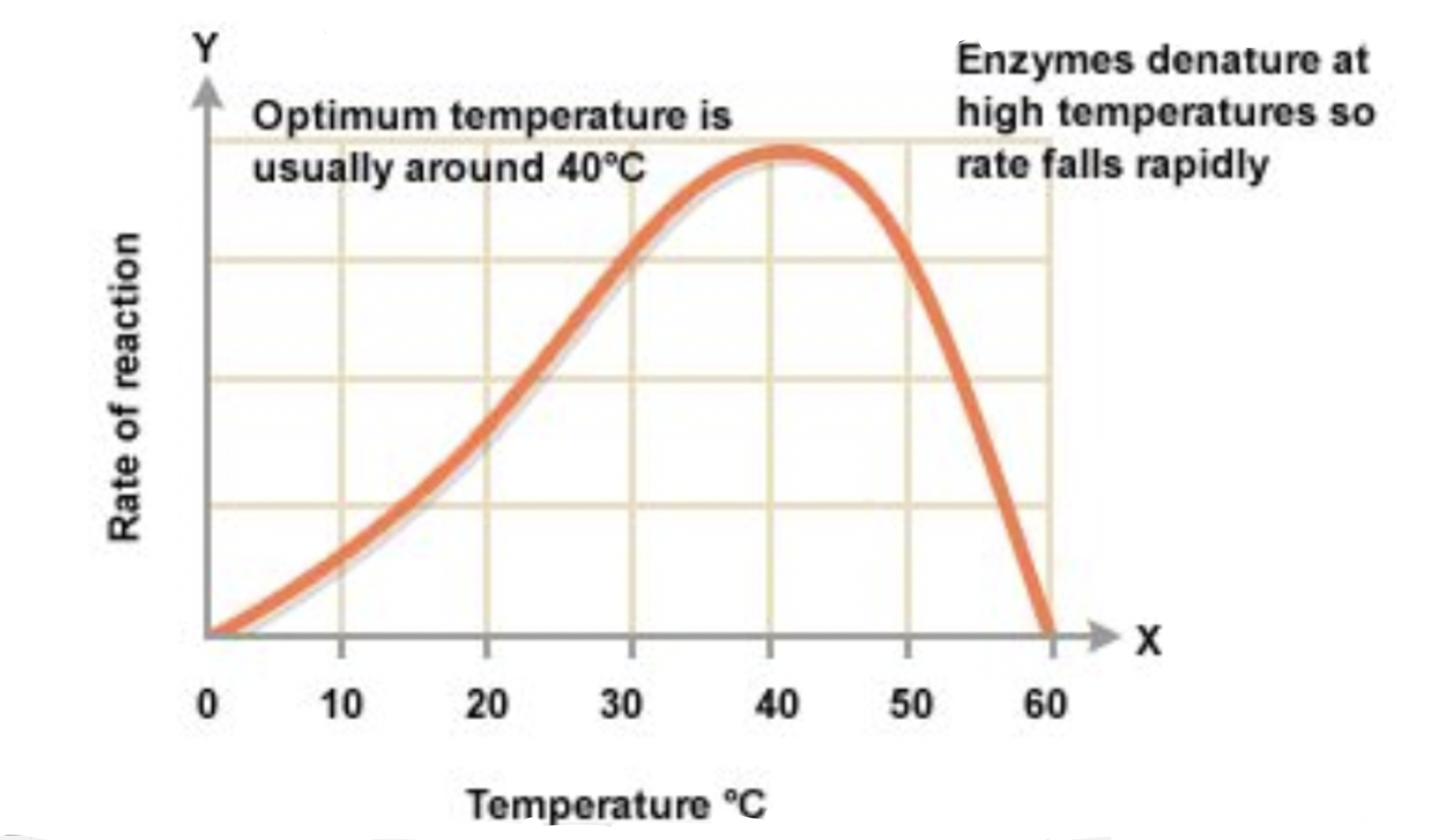
Small proteins denatured by heat or chemicals usually return to their original shape when the cause is removed
As long as the amino acid sequence must remain intact (primary structure)
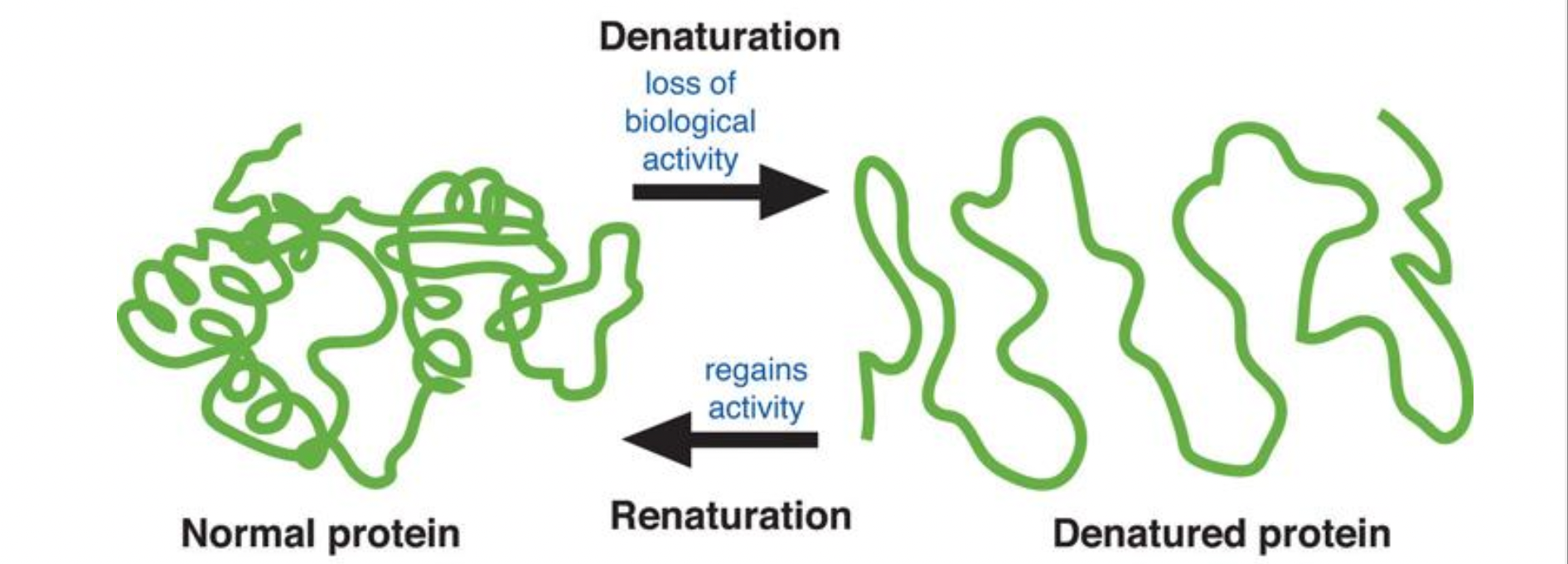
Native conformation = functional form of a protein found under normal biological conditions
Enables recognition to/of other molecules (e.g. enzyme-substrate, antigen-antibody)
When not in native conformation, protein either doesn’t function or works less effectively
Denaturation is the reason that an egg becomes solid when boiled
12-05: Proteins
4 calories per gram of energy → Similar in energy to carbs
Most diverse molecules in living organisms
-- Structural support (muscle)
-- Storage (center of glycogen)
-- Transport (hemoglobin; on RBC which transport oxygen)
-- Signalling (hormones and steroids)
-- Cell response (receptor proteins)
-- Movement (contractile proteins)
-- Defense (antibodies, latching onto antigens and identifying pathogens and things that shouldn’t be there)
-- Catalysis of reactions (enzymes)
-- and more!
The body can’t store protein
Extra protein is converted to energy and/or stored as fat (triglycerides, anything in excess is converted to triglycerides)
Proteins aren’t mostly our main source of energy, but we need their amino acids
Amino Acids
Monomers of proteins (building blocks of proteins)
They are amphiprotic - they have both an acidic and basic component
The carboxyl group is acidic and the amino group is basic
There are 20 different side chains and thus 20 different amino acids
9 are essential amino acids which mean that we need them in our food (must be consumed) because our bodies cannot manufacture them ourselves



Non polar: are non polar because of ∆EN
C–H and C–C bonds are non polar
Polar: polar because of their functional groups
more hydrophilic
SH on cysteine is a functional group called sulfhydryl – its polarity is debated, it is very weakly polar covalent though some consider it non polar
Creates bonds with other cysteine
Electrically charged: side groups are charged
charged carboxyl = negative charge
charged amino = positive charge
Amino acids to know how to draw

Amino acids to note
Cysteine
Contains S in side chain sulfhydryl FG
The S forms disulfide bridges (bonds) with other cysteines
Helps hold chains together
2 cysteines close enough will bond


Proline
Side chain forms a ring with itself
Side chain bonds to base/foundation of the molecule
Causes bends in the polypeptide chain
Proline kink = a bend in the strand


Amino acid formation
Monomers (amino acids) attach to each other by forming peptide bonds
They form these peptide bonds between the amino group of one amino acid and the carboxyl group of another amino acid


A protein is hundreds of amino acids strung together

When counting amino acids, count by side chain (every amino acid has one side chain)

Polypeptides
When over 50 amino acids are strung together it forms a polypeptide (many = poly)
The order of amino acids is determined by an organism’s DNA (instruction manual)
Once the polypeptide chain has been formed, it has to be folded into a specific shape in order to properly function
Structure determines function
If there is something wrong with the DNA then an improper protein will be made. Cystic fibrosis is caused by a single protein being wrong
4 levels of folding (only the first 3 are required to reach full protein status)
Primary structure: sequence of amino acids (aka residues)
Secondary structure: coils and folds
Tertiary structure: supercoiling
Quaternary structure: subunit interaction
Primary Structure
Starts at the N (amino) terminus and ends at the C (carboxyl) terminus
Order of amino acids determines how the protein will be folded
A single mistake can cause a huge change in a protein (e.g. sickle cell anemic which causes the RBC to be half moon shaped and causing them to get stuck)
A single mistake in DNA causes a single amino acid in the protein sequence to be different, leading to a serious change in the shape of RBC

Secondary Structure
Coils and folds
Stabilized by Hydrogen bonding between amino and carboxyl groups
α-helices

H bonding is forming amino groups to other carboxyl groups
Forms a coil
The carboxyl bonds to amino ~4 carbons away
β-pleated sheets
Carboxyl and amino
Instead of the strand staying straight, it will turn a corner and make an S-like curve
Different parts of the protein can coil and sheet (as seen in the bottom image)

Tertiary Structure
Super coiling occurs at the tertiary level when side groups (R) on the amino acids interact with each other and cause another level of folding
Disulfide bridges (if there is a cysteine amino acid)
Hydrophobic interactions (non polar)
Hydrogen bonding (different in tertiary structure, between side groups (as opposed to the base of an amino acid in secondary))
Ionic bonding (between acidic and basic)
Can be a fully functioning protein at this level
Can be a fibre or globular

Quaternary Structure
Not all proteins have this -- if a protein is made up of more than 1 polypeptide strand
Some proteins are made of more than one polypeptide (2 or more subunits interacting)
Hemoglobin: has quaternary structure because one hemoglobin is made of 4 polypeptide chains together
Has an accessory molecule – vitamins and minerals: this is called heme and it is made of iron (Fe) → prosthetic group
Some proteins with quaternary structures also have prosthetic groups (non protein components)
Hemoglobin: 4 heme groups which carries 4 oxygen
People with anemia faint, are pale, and are tired. They take iron supplements to attract the oxygen

Summary

Types of proteins
Globular
One or more polypeptide chains that take on a round spherical shape (forms a blob)
E.g. antibodies, enzymes
Hemoglobin are globular
Can be one or many chains
Fibrous
Long strands or sheets of polypeptide chains
E.g. actin and myosin (muscle contractions), silk, collagen (skin), and keratin (hair)
Pile up to form fibrous tissue (doesn’t ball up, more of a linear structure)
Changing of proteins
Proteins can only function in their optimal shape
Their shape can be impacted by their environment
The 3D shape of a protein can be changed by changes in temperature, pH, or ionic concentration
The loss of shape is called denaturation (if it isn’t in its native conformation/optimal shape)
Therefore proteins have optimal ranges for temperature, pH, and ionic concentration within the function’
Can be rendered useless if the shape is too altered and cannot be reversed if the primary structure is altered (if slightly changed it can still be sort of useless)

Small proteins denatured by heat or chemicals usually return to their original shape when the cause is removed
As long as the amino acid sequence must remain intact (primary structure)

Native conformation = functional form of a protein found under normal biological conditions
Enables recognition to/of other molecules (e.g. enzyme-substrate, antigen-antibody)
When not in native conformation, protein either doesn’t function or works less effectively
Denaturation is the reason that an egg becomes solid when boiled
 Knowt
Knowt