Bonds
Covalent Bonds
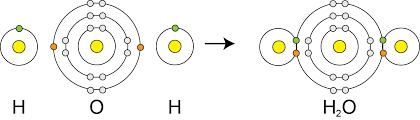
Covalent bonds are formed when 2 non-metal atoms share pairs of electron. Covalent bonds are strong because the shared electron is attracted to both atoms.
Small Molecule |
|
|---|---|
Large Molecule | Polymers |
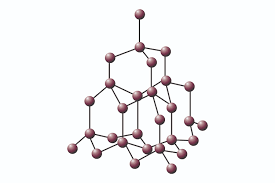
Giant Covalent Bond |
|
Ionic Bonds
Ionic bonds is when metals react with non-metals, electrons are transferred, forming ions.
Naming Compounds-
Ionic compounds from two different elements end in '-ide
Ionic compounds from 3 or more different elements end in -ate.
Metallic Bonds
Metallic bonds are the electrostatic attractions between positive ions and negative delocalised electrons.
Bonds
Covalent Bonds
Covalent bonds are formed when 2 non-metal atoms share pairs of electron. Covalent bonds are strong because the shared electron is attracted to both atoms.
Small Molecule |
|
|---|---|
Large Molecule | Polymers |
Giant Covalent Bond |
|
Ionic Bonds
Ionic bonds is when metals react with non-metals, electrons are transferred, forming ions.
Naming Compounds-
Ionic compounds from two different elements end in '-ide
Ionic compounds from 3 or more different elements end in -ate.
Metallic Bonds
Metallic bonds are the electrostatic attractions between positive ions and negative delocalised electrons.
 Knowt
Knowt