
Ms yuens revision lesson
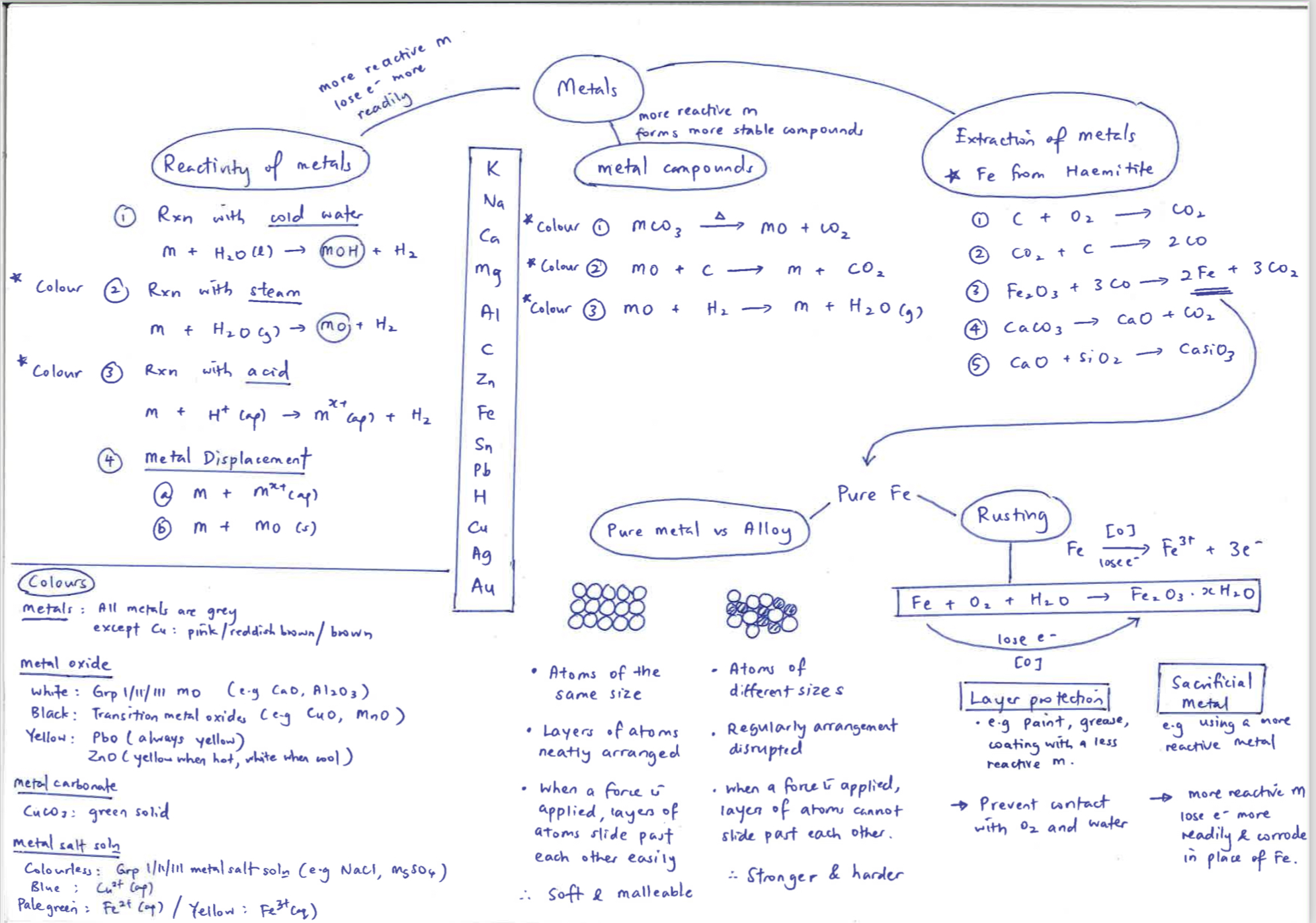
Reaction of reactivity water
metal + water: Ca + 2 H2O → Ca(OH)2+ H2
Zn + 2Hcl → ZnCl2 +H2
Fe + H2O(g) -> FeO + H2
Pb + CuSO4 - > PbSO4 + Cu
Fe+CuO →FeO + Cu
Na2CO3 →
|_ Na is a very reactive M, hence Na2CO3 is very thermally stable , hence it cannot decompose on heating
ZnCO3 → ZnO + CO2
Au2CO3 → 4Au + O2 + 2 CO2
2 PbO + C → 2 Pb + CO2
FeO + H2 → Fe + H2O
first 4 can react with water
steam can react until iron , can form
all until hydrogen can react with
Zn - Au can be reduced by carbon
K please
Na stop
_______________
Ca calling
Mg me
______________
Al a
________________
C cool
________________
Zn Zebra
Fe I
Sn Totally
Pb Love
H Happy
Cu Crescent
Ag School
Au. Girls
aluminium is a special metal that is rarely use in reaction, as there is a layer of aluminium oxide
Ms yuens revision lesson
Reaction of reactivity water
metal + water: Ca + 2 H2O → Ca(OH)2+ H2
Zn + 2Hcl → ZnCl2 +H2
Fe + H2O(g) -> FeO + H2
Pb + CuSO4 - > PbSO4 + Cu
Fe+CuO →FeO + Cu
Na2CO3 →
|_ Na is a very reactive M, hence Na2CO3 is very thermally stable , hence it cannot decompose on heating
ZnCO3 → ZnO + CO2
Au2CO3 → 4Au + O2 + 2 CO2
2 PbO + C → 2 Pb + CO2
FeO + H2 → Fe + H2O
first 4 can react with water
steam can react until iron , can form
all until hydrogen can react with
Zn - Au can be reduced by carbon
K please
Na stop
_______________
Ca calling
Mg me
______________
Al a
________________
C cool
________________
Zn Zebra
Fe I
Sn Totally
Pb Love
H Happy
Cu Crescent
Ag School
Au. Girls

aluminium is a special metal that is rarely use in reaction, as there is a layer of aluminium oxide
 Knowt
Knowt