
Mineral Nutrition
Methods to Study the Mineral Requirements of Plants:
In 1860, Julius von Sachs, a prominent German botanist, demonstrated, for the first time, that plants could be grown to maturity in a defined nutrient solution in the complete absence of soil.
This technique of growing plants in a nutrient solution is known as hydroponics.
Since then, a number of improvised methods have been employed to try and determine the mineral nutrients essential for plants.
The essence of all these methods involves the culture of plants in a soil-free, defined mineral solution.
These methods require purified water and mineral nutrient salts.
After a series of experiments in which the roots of the plants were immersed in nutrient solutions and wherein an element was added/substituted/removed or given in varying concentrations, a mineral solution.
Essential Mineral Elements:
Most of the minerals present in soil can enter plants through roots.
In fact, more than sixty elements of the 105 discovered so far are found in different plants.
Some plant species accumulate selenium, some others gold, while some plants growing near nuclear test sites take up radioactive strontium.
There are techniques that are able to detect the minerals even at a very low concentration (10^-8 g/ mL).
Criteria for Essentiality:
The criteria for the essentiality of an element are given below:
The element must be absolutely necessary for supporting normal growth and reproduction.
In the absence of the element, the plants do not complete their life cycle or set the seeds.
The requirement of the element must be specific and not replaceable by another element.
In other words, deficiency of any one element cannot be met by supplying some other element.
The element must be directly involved in the metabolism of the plant.
Based upon the above criteria only a few elements have been found to be absolutely essential for plant growth and metabolism.
These elements are further divided into two broad categories based on their quantitative requirements.
Macronutrients are generally present in plant tissues in large amounts (in excess of 10 mmole Kg –1 of dry matter).
The macronutrients include carbon, hydrogen, oxygen, nitrogen, phosphorous, sulfur, potassium, calcium, and magnesium.
Of these, carbon, hydrogen, and oxygen are mainly obtained from CO2 and H2O, while the others are absorbed from the soil as mineral nutrition.
Micronutrients or trace elements are needed in very small amounts (less than 10 mmole Kg –1 of dry matter).
These include iron, manganese, copper, molybdenum, zinc, boron, chlorine, and nickel.
In addition to the 17 essential elements named above, there are some beneficial elements such as sodium, silicon, cobalt, and selenium.
They are required by higher plants.
Essential elements can also be grouped into four broad categories on the basis of their diverse functions.
These categories are:
Essential elements as components of biomolecules and hence structural elements of cells (e.g., carbon, hydrogen, oxygen, and nitrogen).
Essential elements that are components of energy-related chemical compounds in plants (e.g., magnesium in chlorophyll and phosphorous in ATP).
Essential elements that activate or inhibit enzymes, for example, Mg2+ is an activator for both ribulose bisphosphate carboxylase oxygenase and phosphoenol pyruvate carboxylase, both of which are critical enzymes in photosynthetic carbon fixation; Zn2+ is an activator of alcohol dehydrogenase and Mo of nitrogenase during nitrogen metabolism.
Some essential elements can alter the osmotic potential of a cell.
Potassium plays an important role in the opening and closing of stomata.
Role of Macro- and Micro-nutrients:
Essential elements perform several functions.
They participate in various metabolic processes in the plant cells such as permeability of cell membrane, maintenance of osmotic concentration of cell sap, electron-transport systems, buffering action, enzymatic activity, and act as major constituents of macromolecules and co-enzymes.
Various forms and functions of essential nutrient elements are as follows.
Nitrogen:
This is the essential nutrient element required by plants in the greatest amount.
It is absorbed mainly as NO3 – though some are also taken up as NO2 – or NH4+.
Nitrogen is required by all parts of a plant, particularly the meristematic tissues and the metabolically active cells.
Nitrogen is one of the major constituents of proteins, nucleic acids, vitamins, and hormones.
Phosphorus:
Phosphorus is absorbed by the plants from the soil in the form of phosphate ions (either as HPO2 4 − or HPO4 2−).
Phosphorus is a constituent of cell membranes, certain proteins, all nucleic acids, and nucleotides, and is required for all phosphorylation reactions.
Potassium:
It is absorbed as a potassium ion (K+ ).
In plants, this is required in more abundant quantities in the meristematic tissues, buds, leaves, and root tips.
Potassium helps to maintain an anion-cation balance in cells and is involved in protein synthesis, opening and closing of stomata, activation of enzymes, and the maintenance of the turgidity of cells.
Calcium:
The plant absorbs calcium from the soil in the form of calcium ions (Ca2+).
Calcium is required by meristematic and differentiating tissues.
During cell division, it is used in the synthesis of the cell walls, particularly as calcium pectate in the middle lamella.
It is also needed during the formation of the mitotic spindle.
It accumulates in older leaves.
It is involved in the normal functioning of the cell membranes.
It activates certain enzymes and plays an important role in regulating metabolic activities.
Magnesium:
It is absorbed by plants in the form of divalent Mg2+.
It activates the enzymes of respiration, and photosynthesis and is involved in the synthesis of DNA and RNA.
Magnesium is a constituent of the ring structure of chlorophyll and helps to maintain the ribosome structure.
Sulfur:
Plants obtain sulfur in the form of sulfate ( SO4 2−).
Sulfur is present in two amino acids – cysteine and methionine and is the main constituent of several coenzymes, vitamins (thiamine, biotin, Coenzyme A), and ferredoxin.
Iron:
Plants obtain iron in the form of ferric ions (Fe3+).
It is required in larger amounts in comparison to other micronutrients.
It is an important constituent of proteins involved in the transfer of electrons like ferredoxin and cytochromes.
It is reversibly oxidized from Fe2+ to Fe3+ during electron transfer.
It activates the catalase enzyme and is essential for the formation of chlorophyll.
Manganese:
It is absorbed in the form of manganous ions (Mn2+).
It activates many enzymes involved in photosynthesis, respiration, and nitrogen metabolism.
The best-defined function of manganese is in the splitting of water to liberate oxygen during photosynthesis.
Zinc:
Plants obtain zinc as Zn2+ ions.
It activates various especially carboxylases.
It is also needed in the synthesis of auxin.
Copper:
It is absorbed as cupric ions (Cu2+).
It is essential for the overall metabolism of plants.
Like iron, it is associated with certain enzymes involved in redox reactions and is reversibly oxidized from Cu+ to Cu2+.
Boron:
It is absorbed as BO3 3− or B O4 7 2−.
Boron is required for the uptake and utilization of Ca2+, membrane functioning, pollen germination, cell elongation, cell differentiation, and carbohydrate translocation.
Molybdenum:
Plants obtain it in the form of molybdate ions (MoO2 2+).
It is a component of several enzymes, including nitrogenase and nitrate reductase both of which participate in nitrogen metabolism.
Chlorine:
It is absorbed in the form of chloride anion (Cl– ).
Along with Na+ and K+, it helps in determining the solute concentration and the anion cation balance in cells.
It is essential for the water-splitting reaction in photosynthesis, a reaction that leads to oxygen evolution.
Deficiency Symptoms of Essential Elements:
Whenever the supply of an essential element becomes limited, plant growth is retarded.
The concentration of the essential element below which plant growth is retarded is termed critical concentration.
The element is said to be deficient when present below the critical concentration.
Since each element has one or more specific structural or functional roles in plants, in the absence of any particular element, plants show certain morphological changes.
These morphological changes are indicative of certain element deficiencies and are called deficiency symptoms.
The deficiency symptoms vary from element to element and they disappear when the deficient mineral nutrient is provided to the plant.
However, if deprivation continues, it may eventually lead to the death of the plant.
The parts of the plants that show the deficiency symptoms also depend on the mobility of the element in the plant.
For elements that are actively mobilized within the plants and exported to young developing tissues, the deficiency symptoms tend to appear first in the older tissues.
For example, the deficiency symptoms of nitrogen, potassium, and magnesium are visible first in the senescent leaves.
In the older leaves, biomolecules containing these elements are broken down, making these elements available for mobilizing to younger leaves.
The deficiency symptoms tend to appear first in the young tissues whenever the elements are relatively immobile and are not transported out of the mature organs, for example, elements like sulfur and calcium are a part of the structural component of the cell and hence are not easily released.
This aspect of mineral nutrition of plants is of great significance and importance to agriculture and horticulture.
The kind of deficiency symptoms shown in plants includes chlorosis, necrosis, stunted plant growth, premature fall of leaves and buds, and inhibition of cell division.
Chlorosis is the loss of chlorophyll leading to yellowing in leaves.
This symptom is caused by the deficiency of elements N, K, Mg, S, Fe, Mn, Zn, and Mo.
Likewise, necrosis, or death of tissue, particularly leaf tissue, is due to the deficiency of Ca, Mg, Cu, and K.
Lack or low levels of N, K, S, and Mo cause inhibition of cell division.
Some elements like N, S, and Mo delay flowering if their concentration in plants is low.
Toxicity of Micronutrients:
The requirement of micronutrients is always in low amounts while their moderate decrease causes the deficiency symptoms and a moderate increase causes toxicity.
In other words, there is a narrow range of concentration at which the elements are optimum.
Any mineral ion concentration in tissues that reduces the dry weight of tissues by about 10 percent is considered toxic.
Such critical concentrations vary widely among different micronutrients.
The toxicity symptoms are difficult to identify.
Toxicity levels for any element also vary for different plants.
Many times, an excess of an element may inhibit the uptake of another element.
For example, the prominent symptom of manganese toxicity is the appearance of brown spots surrounded by chlorotic veins.
It is important to know that manganese competes with iron and magnesium for uptake and with magnesium for binding with enzymes.
Manganese also inhibits calcium translocation in the shoot apex.
Therefore, excess manganese may, in fact, induce deficiencies in iron, magnesium, and calcium.
Thus, what appears as symptoms of manganese toxicity may actually be the deficiency symptoms of iron, magnesium, and calcium.
Mechanism of Absorption of Elements:
Much of the studies on the mechanism of absorption of elements by plants have been carried out in isolated cells, tissues, or organs.
These studies revealed that the process of absorption can be demarcated into two main phases.
In the first phase, the initial rapid uptake of ions into the ‘free space’ or ‘outer space’ of cells – the apoplast, is passive. In the second phase of uptake, the ions are taken in slowly into the ‘inner space’ – the symplast of the cells.
The passive movement of ions into the apoplast usually occurs through ion channels, the trans-membrane proteins that function as selective pores.
On the other hand, the entry or exit of ions to and from the symplast requires the expenditure of metabolic energy, which is an active process.
The movement of ions is usually called flux; the inward movement into the cells is in flux and the outward movement, is efflux.
Translocation of Solutes:
Mineral salts are translocated through the xylem along with the ascending stream of water, which is pulled up through the plant by transpirational pull.
Analysis of xylem sap shows the presence of mineral salts in it.
Soil as Reservoir of Essential Elements:
The majority of the nutrients that are essential for the growth and development of plants become available to the roots due to weathering and the breakdown of rocks.
These processes enrich the soil with dissolved ions and inorganic salts.
Since they are derived from rock minerals, their role in plant nutrition is referred to as mineral nutrition.
Soil consists of a wide variety of substances.
Soil not only supplies minerals but also harbors nitrogen-fixing bacteria, and other microbes hold water, supply air to the roots, and act as a matrix that stabilizes the plant.
Since deficiency of essential minerals affects crop yield, there is often a need for supplying them through fertilizers.
Both macro-nutrients (N, P, K, S, etc.) and micro-nutrients (Cu, Zn, Fe, Mn, etc.) form components of fertilizers and are applied as per need.
Metabolism of Nitrogen:
Nitrogen Cycle:
Apart from carbon, hydrogen, and oxygen, nitrogen is the most prevalent element in living organisms.
Nitrogen is a constituent of amino acids, proteins, hormones, chlorophyll, and many vitamins.
Plants compete with microbes for the limited nitrogen that is available in the soil.
Thus, nitrogen is a limiting nutrient for both natural and agricultural ecosystems.
Nitrogen exists as two nitrogen atoms joined by a very strong triple covalent bond (N ≡ N).
The process of conversion of nitrogen (N2 ) to ammonia is termed nitrogen fixation.
In nature, lightning and ultraviolet radiation provide enough energy to convert nitrogen to nitrogen oxides (NO, NO2, N2O).
Industrial combustions, forest fires, automobile exhausts, and power-generating stations are also sources of atmospheric nitrogen oxides.
The decomposition of organic nitrogen of dead plants and animals into ammonia is called ammonification.
Some of this ammonia volatilizes and re-enters the atmosphere but most of it is converted into nitrate by soil bacteria in the following steps:
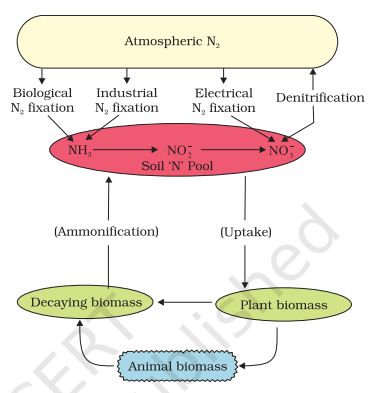
Ammonia is first oxidized to nitrite by the bacteria Nitrosomonas and/or Nitrococcus.
The nitrite is further oxidized to nitrate with the help of the bacterium Nitrobacter.
These steps are called nitrification.
These nitrifying bacteria are chemoautotrophs.
The nitrate thus formed is absorbed by plants and is transported to the leaves.
In leaves, it is reduced to form ammonia which finally forms the amine group of amino acids.
Nitrate present in the soil is also reduced to nitrogen by the process of denitrification.
Denitrification is carried by the bacteria Pseudomonas and Thiobacillus.
Biological Nitrogen Fixation:
Very few living organisms can utilize nitrogen in the form of N2, available abundantly in the air.
Only certain prokaryotic species are capable of fixing nitrogen.
The reduction of nitrogen to ammonia by living organisms is called biological nitrogen fixation.
The enzyme, nitrogenase which is capable of nitrogen reduction is present exclusively in prokaryotes.
Such microbes are called N2 - fixers.
The nitrogen-fixing microbes could be free-living or symbiotic.
Examples of free-living nitrogen-fixing aerobic microbes are Azotobacter and Beijerinckia while Rhodospirillum is anaerobic and free-living.
In addition, a number of cyanobacteria such as Anabaena and Nostoc are also free-living nitrogen-fixers.
Symbiotic biological nitrogen fixation:
Several types of symbiotic biological nitrogen-fixing associations are known.
The most prominent among them is the legume-bacteria relationship.
Species of rod-shaped Rhizobium have a relationship with the roots of several legumes such as alfalfa, sweet clover, sweet pea, lentils, garden pea, broad bean, clover beans, etc.
The most common association with roots is nodules.
These nodules are small outgrowths on the roots.
The microbe, Frankia, also produces nitrogen-fixing nodules on the roots of nonleguminous plants (e.g., Alnus).
Both Rhizobium and Frankia are free-living in soil, but as symbionts, can fix atmospheric nitrogen.
Nodule Formation:
Nodule formation involves a sequence of multiple interactions between Rhizobium and the roots of the host plant.
Principal stages in the nodule formation are summarised as follows:
Rhizobia multiply and colonise the surroundings of roots and get attached to epidermal and root hair cells.
The root-hairs curl and the bacteria invade the root hair.
An infection thread is produced carrying the bacteria into the cortex of the root, where they initiate the nodule formation in the cortex of the root.
Then the bacteria are released from the thread into the cells which leads to the differentiation of specialized nitrogen-fixing cells.
The nodule thus formed, establishes a direct vascular connection with the host for the exchange of nutrients.
The nodule contains all the necessary biochemical components, such as the enzyme nitrogenase and leghaemoglobin.
The enzyme nitrogenase is a Mo-Fe protein and catalyzes the conversion of atmospheric nitrogen to ammonia, the first stable product of nitrogen fixation.
The enzyme nitrogenase is highly sensitive to molecular oxygen; it requires anaerobic conditions.
The nodules have adaptations that ensure that the enzyme is protected from oxygen.
To protect these enzymes, the nodule contains an oxygen scavenger called leghemoglobin.
It is interesting to note that these microbes live as aerobes under free-living conditions (where nitrogenase is not operational), but during nitrogen-fixing events, they become anaerobic (thus protecting the nitrogenase enzyme).
The ammonia synthesis by nitrogenase requires a very high input of energy (8 ATP for each NH3 produced).
The energy required, thus, is obtained from the respiration of the host cells.
The fate of ammonia:
At physiological pH, the ammonia is protonated to form NH4 + (ammonium) ion.
While most plants can assimilate nitrate as well as ammonium ions, the latter is quite toxic to plants and hence cannot accumulate in them
There are two main ways in which this can take place:
Reductive amination: In these processes, ammonia reacts with α-ketoglutaric acid and forms glutamic acid.
Transamination: It involves the transfer of amino groups from one amino acid to the keto group of a keto acid.
Glutamic acid is the main amino acid from which the transfer of NH2, the amino group takes place and other amino acids are formed through transamination.
The enzyme transaminase catalyzes all such reactions.
The two most important amides – asparagine and glutamine – found in plants are a structural part of proteins.
They are formed from two amino acids, namely aspartic acid and glutamic acid, respectively, by the addition of another amino group to each.
The hydroxyl part of the acid is replaced by another NH2 – radicle.
Since amides contain more nitrogen than amino acids, they are transported to other parts of the plant via xylem vessels.
In addition, along with the transpiration stream the nodules of some plants (e.g., soybean) export the fixed nitrogen as ureides.
These compounds also have a particularly high nitrogen-to-carbon ratio.
Mineral Nutrition
Methods to Study the Mineral Requirements of Plants:
In 1860, Julius von Sachs, a prominent German botanist, demonstrated, for the first time, that plants could be grown to maturity in a defined nutrient solution in the complete absence of soil.
This technique of growing plants in a nutrient solution is known as hydroponics.
Since then, a number of improvised methods have been employed to try and determine the mineral nutrients essential for plants.
The essence of all these methods involves the culture of plants in a soil-free, defined mineral solution.
These methods require purified water and mineral nutrient salts.
After a series of experiments in which the roots of the plants were immersed in nutrient solutions and wherein an element was added/substituted/removed or given in varying concentrations, a mineral solution.
Essential Mineral Elements:
Most of the minerals present in soil can enter plants through roots.
In fact, more than sixty elements of the 105 discovered so far are found in different plants.
Some plant species accumulate selenium, some others gold, while some plants growing near nuclear test sites take up radioactive strontium.
There are techniques that are able to detect the minerals even at a very low concentration (10^-8 g/ mL).
Criteria for Essentiality:
The criteria for the essentiality of an element are given below:
The element must be absolutely necessary for supporting normal growth and reproduction.
In the absence of the element, the plants do not complete their life cycle or set the seeds.
The requirement of the element must be specific and not replaceable by another element.
In other words, deficiency of any one element cannot be met by supplying some other element.
The element must be directly involved in the metabolism of the plant.
Based upon the above criteria only a few elements have been found to be absolutely essential for plant growth and metabolism.
These elements are further divided into two broad categories based on their quantitative requirements.
Macronutrients are generally present in plant tissues in large amounts (in excess of 10 mmole Kg –1 of dry matter).
The macronutrients include carbon, hydrogen, oxygen, nitrogen, phosphorous, sulfur, potassium, calcium, and magnesium.
Of these, carbon, hydrogen, and oxygen are mainly obtained from CO2 and H2O, while the others are absorbed from the soil as mineral nutrition.
Micronutrients or trace elements are needed in very small amounts (less than 10 mmole Kg –1 of dry matter).
These include iron, manganese, copper, molybdenum, zinc, boron, chlorine, and nickel.
In addition to the 17 essential elements named above, there are some beneficial elements such as sodium, silicon, cobalt, and selenium.
They are required by higher plants.
Essential elements can also be grouped into four broad categories on the basis of their diverse functions.
These categories are:
Essential elements as components of biomolecules and hence structural elements of cells (e.g., carbon, hydrogen, oxygen, and nitrogen).
Essential elements that are components of energy-related chemical compounds in plants (e.g., magnesium in chlorophyll and phosphorous in ATP).
Essential elements that activate or inhibit enzymes, for example, Mg2+ is an activator for both ribulose bisphosphate carboxylase oxygenase and phosphoenol pyruvate carboxylase, both of which are critical enzymes in photosynthetic carbon fixation; Zn2+ is an activator of alcohol dehydrogenase and Mo of nitrogenase during nitrogen metabolism.
Some essential elements can alter the osmotic potential of a cell.
Potassium plays an important role in the opening and closing of stomata.
Role of Macro- and Micro-nutrients:
Essential elements perform several functions.
They participate in various metabolic processes in the plant cells such as permeability of cell membrane, maintenance of osmotic concentration of cell sap, electron-transport systems, buffering action, enzymatic activity, and act as major constituents of macromolecules and co-enzymes.
Various forms and functions of essential nutrient elements are as follows.
Nitrogen:
This is the essential nutrient element required by plants in the greatest amount.
It is absorbed mainly as NO3 – though some are also taken up as NO2 – or NH4+.
Nitrogen is required by all parts of a plant, particularly the meristematic tissues and the metabolically active cells.
Nitrogen is one of the major constituents of proteins, nucleic acids, vitamins, and hormones.
Phosphorus:
Phosphorus is absorbed by the plants from the soil in the form of phosphate ions (either as HPO2 4 − or HPO4 2−).
Phosphorus is a constituent of cell membranes, certain proteins, all nucleic acids, and nucleotides, and is required for all phosphorylation reactions.
Potassium:
It is absorbed as a potassium ion (K+ ).
In plants, this is required in more abundant quantities in the meristematic tissues, buds, leaves, and root tips.
Potassium helps to maintain an anion-cation balance in cells and is involved in protein synthesis, opening and closing of stomata, activation of enzymes, and the maintenance of the turgidity of cells.
Calcium:
The plant absorbs calcium from the soil in the form of calcium ions (Ca2+).
Calcium is required by meristematic and differentiating tissues.
During cell division, it is used in the synthesis of the cell walls, particularly as calcium pectate in the middle lamella.
It is also needed during the formation of the mitotic spindle.
It accumulates in older leaves.
It is involved in the normal functioning of the cell membranes.
It activates certain enzymes and plays an important role in regulating metabolic activities.
Magnesium:
It is absorbed by plants in the form of divalent Mg2+.
It activates the enzymes of respiration, and photosynthesis and is involved in the synthesis of DNA and RNA.
Magnesium is a constituent of the ring structure of chlorophyll and helps to maintain the ribosome structure.
Sulfur:
Plants obtain sulfur in the form of sulfate ( SO4 2−).
Sulfur is present in two amino acids – cysteine and methionine and is the main constituent of several coenzymes, vitamins (thiamine, biotin, Coenzyme A), and ferredoxin.
Iron:
Plants obtain iron in the form of ferric ions (Fe3+).
It is required in larger amounts in comparison to other micronutrients.
It is an important constituent of proteins involved in the transfer of electrons like ferredoxin and cytochromes.
It is reversibly oxidized from Fe2+ to Fe3+ during electron transfer.
It activates the catalase enzyme and is essential for the formation of chlorophyll.
Manganese:
It is absorbed in the form of manganous ions (Mn2+).
It activates many enzymes involved in photosynthesis, respiration, and nitrogen metabolism.
The best-defined function of manganese is in the splitting of water to liberate oxygen during photosynthesis.
Zinc:
Plants obtain zinc as Zn2+ ions.
It activates various especially carboxylases.
It is also needed in the synthesis of auxin.
Copper:
It is absorbed as cupric ions (Cu2+).
It is essential for the overall metabolism of plants.
Like iron, it is associated with certain enzymes involved in redox reactions and is reversibly oxidized from Cu+ to Cu2+.
Boron:
It is absorbed as BO3 3− or B O4 7 2−.
Boron is required for the uptake and utilization of Ca2+, membrane functioning, pollen germination, cell elongation, cell differentiation, and carbohydrate translocation.
Molybdenum:
Plants obtain it in the form of molybdate ions (MoO2 2+).
It is a component of several enzymes, including nitrogenase and nitrate reductase both of which participate in nitrogen metabolism.
Chlorine:
It is absorbed in the form of chloride anion (Cl– ).
Along with Na+ and K+, it helps in determining the solute concentration and the anion cation balance in cells.
It is essential for the water-splitting reaction in photosynthesis, a reaction that leads to oxygen evolution.
Deficiency Symptoms of Essential Elements:
Whenever the supply of an essential element becomes limited, plant growth is retarded.
The concentration of the essential element below which plant growth is retarded is termed critical concentration.
The element is said to be deficient when present below the critical concentration.
Since each element has one or more specific structural or functional roles in plants, in the absence of any particular element, plants show certain morphological changes.
These morphological changes are indicative of certain element deficiencies and are called deficiency symptoms.
The deficiency symptoms vary from element to element and they disappear when the deficient mineral nutrient is provided to the plant.
However, if deprivation continues, it may eventually lead to the death of the plant.
The parts of the plants that show the deficiency symptoms also depend on the mobility of the element in the plant.
For elements that are actively mobilized within the plants and exported to young developing tissues, the deficiency symptoms tend to appear first in the older tissues.
For example, the deficiency symptoms of nitrogen, potassium, and magnesium are visible first in the senescent leaves.
In the older leaves, biomolecules containing these elements are broken down, making these elements available for mobilizing to younger leaves.
The deficiency symptoms tend to appear first in the young tissues whenever the elements are relatively immobile and are not transported out of the mature organs, for example, elements like sulfur and calcium are a part of the structural component of the cell and hence are not easily released.
This aspect of mineral nutrition of plants is of great significance and importance to agriculture and horticulture.
The kind of deficiency symptoms shown in plants includes chlorosis, necrosis, stunted plant growth, premature fall of leaves and buds, and inhibition of cell division.
Chlorosis is the loss of chlorophyll leading to yellowing in leaves.
This symptom is caused by the deficiency of elements N, K, Mg, S, Fe, Mn, Zn, and Mo.
Likewise, necrosis, or death of tissue, particularly leaf tissue, is due to the deficiency of Ca, Mg, Cu, and K.
Lack or low levels of N, K, S, and Mo cause inhibition of cell division.
Some elements like N, S, and Mo delay flowering if their concentration in plants is low.
Toxicity of Micronutrients:
The requirement of micronutrients is always in low amounts while their moderate decrease causes the deficiency symptoms and a moderate increase causes toxicity.
In other words, there is a narrow range of concentration at which the elements are optimum.
Any mineral ion concentration in tissues that reduces the dry weight of tissues by about 10 percent is considered toxic.
Such critical concentrations vary widely among different micronutrients.
The toxicity symptoms are difficult to identify.
Toxicity levels for any element also vary for different plants.
Many times, an excess of an element may inhibit the uptake of another element.
For example, the prominent symptom of manganese toxicity is the appearance of brown spots surrounded by chlorotic veins.
It is important to know that manganese competes with iron and magnesium for uptake and with magnesium for binding with enzymes.
Manganese also inhibits calcium translocation in the shoot apex.
Therefore, excess manganese may, in fact, induce deficiencies in iron, magnesium, and calcium.
Thus, what appears as symptoms of manganese toxicity may actually be the deficiency symptoms of iron, magnesium, and calcium.
Mechanism of Absorption of Elements:
Much of the studies on the mechanism of absorption of elements by plants have been carried out in isolated cells, tissues, or organs.
These studies revealed that the process of absorption can be demarcated into two main phases.
In the first phase, the initial rapid uptake of ions into the ‘free space’ or ‘outer space’ of cells – the apoplast, is passive. In the second phase of uptake, the ions are taken in slowly into the ‘inner space’ – the symplast of the cells.
The passive movement of ions into the apoplast usually occurs through ion channels, the trans-membrane proteins that function as selective pores.
On the other hand, the entry or exit of ions to and from the symplast requires the expenditure of metabolic energy, which is an active process.
The movement of ions is usually called flux; the inward movement into the cells is in flux and the outward movement, is efflux.
Translocation of Solutes:
Mineral salts are translocated through the xylem along with the ascending stream of water, which is pulled up through the plant by transpirational pull.
Analysis of xylem sap shows the presence of mineral salts in it.
Soil as Reservoir of Essential Elements:
The majority of the nutrients that are essential for the growth and development of plants become available to the roots due to weathering and the breakdown of rocks.
These processes enrich the soil with dissolved ions and inorganic salts.
Since they are derived from rock minerals, their role in plant nutrition is referred to as mineral nutrition.
Soil consists of a wide variety of substances.
Soil not only supplies minerals but also harbors nitrogen-fixing bacteria, and other microbes hold water, supply air to the roots, and act as a matrix that stabilizes the plant.
Since deficiency of essential minerals affects crop yield, there is often a need for supplying them through fertilizers.
Both macro-nutrients (N, P, K, S, etc.) and micro-nutrients (Cu, Zn, Fe, Mn, etc.) form components of fertilizers and are applied as per need.
Metabolism of Nitrogen:
Nitrogen Cycle:
Apart from carbon, hydrogen, and oxygen, nitrogen is the most prevalent element in living organisms.
Nitrogen is a constituent of amino acids, proteins, hormones, chlorophyll, and many vitamins.
Plants compete with microbes for the limited nitrogen that is available in the soil.
Thus, nitrogen is a limiting nutrient for both natural and agricultural ecosystems.
Nitrogen exists as two nitrogen atoms joined by a very strong triple covalent bond (N ≡ N).
The process of conversion of nitrogen (N2 ) to ammonia is termed nitrogen fixation.
In nature, lightning and ultraviolet radiation provide enough energy to convert nitrogen to nitrogen oxides (NO, NO2, N2O).
Industrial combustions, forest fires, automobile exhausts, and power-generating stations are also sources of atmospheric nitrogen oxides.
The decomposition of organic nitrogen of dead plants and animals into ammonia is called ammonification.
Some of this ammonia volatilizes and re-enters the atmosphere but most of it is converted into nitrate by soil bacteria in the following steps:

Ammonia is first oxidized to nitrite by the bacteria Nitrosomonas and/or Nitrococcus.
The nitrite is further oxidized to nitrate with the help of the bacterium Nitrobacter.
These steps are called nitrification.
These nitrifying bacteria are chemoautotrophs.
The nitrate thus formed is absorbed by plants and is transported to the leaves.
In leaves, it is reduced to form ammonia which finally forms the amine group of amino acids.
Nitrate present in the soil is also reduced to nitrogen by the process of denitrification.
Denitrification is carried by the bacteria Pseudomonas and Thiobacillus.
Biological Nitrogen Fixation:
Very few living organisms can utilize nitrogen in the form of N2, available abundantly in the air.
Only certain prokaryotic species are capable of fixing nitrogen.
The reduction of nitrogen to ammonia by living organisms is called biological nitrogen fixation.
The enzyme, nitrogenase which is capable of nitrogen reduction is present exclusively in prokaryotes.
Such microbes are called N2 - fixers.
The nitrogen-fixing microbes could be free-living or symbiotic.
Examples of free-living nitrogen-fixing aerobic microbes are Azotobacter and Beijerinckia while Rhodospirillum is anaerobic and free-living.
In addition, a number of cyanobacteria such as Anabaena and Nostoc are also free-living nitrogen-fixers.
Symbiotic biological nitrogen fixation:
Several types of symbiotic biological nitrogen-fixing associations are known.
The most prominent among them is the legume-bacteria relationship.
Species of rod-shaped Rhizobium have a relationship with the roots of several legumes such as alfalfa, sweet clover, sweet pea, lentils, garden pea, broad bean, clover beans, etc.
The most common association with roots is nodules.
These nodules are small outgrowths on the roots.
The microbe, Frankia, also produces nitrogen-fixing nodules on the roots of nonleguminous plants (e.g., Alnus).
Both Rhizobium and Frankia are free-living in soil, but as symbionts, can fix atmospheric nitrogen.
Nodule Formation:
Nodule formation involves a sequence of multiple interactions between Rhizobium and the roots of the host plant.
Principal stages in the nodule formation are summarised as follows:
Rhizobia multiply and colonise the surroundings of roots and get attached to epidermal and root hair cells.
The root-hairs curl and the bacteria invade the root hair.
An infection thread is produced carrying the bacteria into the cortex of the root, where they initiate the nodule formation in the cortex of the root.
Then the bacteria are released from the thread into the cells which leads to the differentiation of specialized nitrogen-fixing cells.
The nodule thus formed, establishes a direct vascular connection with the host for the exchange of nutrients.
The nodule contains all the necessary biochemical components, such as the enzyme nitrogenase and leghaemoglobin.
The enzyme nitrogenase is a Mo-Fe protein and catalyzes the conversion of atmospheric nitrogen to ammonia, the first stable product of nitrogen fixation.
The enzyme nitrogenase is highly sensitive to molecular oxygen; it requires anaerobic conditions.
The nodules have adaptations that ensure that the enzyme is protected from oxygen.
To protect these enzymes, the nodule contains an oxygen scavenger called leghemoglobin.
It is interesting to note that these microbes live as aerobes under free-living conditions (where nitrogenase is not operational), but during nitrogen-fixing events, they become anaerobic (thus protecting the nitrogenase enzyme).
The ammonia synthesis by nitrogenase requires a very high input of energy (8 ATP for each NH3 produced).
The energy required, thus, is obtained from the respiration of the host cells.
The fate of ammonia:
At physiological pH, the ammonia is protonated to form NH4 + (ammonium) ion.
While most plants can assimilate nitrate as well as ammonium ions, the latter is quite toxic to plants and hence cannot accumulate in them
There are two main ways in which this can take place:
Reductive amination: In these processes, ammonia reacts with α-ketoglutaric acid and forms glutamic acid.
Transamination: It involves the transfer of amino groups from one amino acid to the keto group of a keto acid.
Glutamic acid is the main amino acid from which the transfer of NH2, the amino group takes place and other amino acids are formed through transamination.
The enzyme transaminase catalyzes all such reactions.
The two most important amides – asparagine and glutamine – found in plants are a structural part of proteins.
They are formed from two amino acids, namely aspartic acid and glutamic acid, respectively, by the addition of another amino group to each.
The hydroxyl part of the acid is replaced by another NH2 – radicle.
Since amides contain more nitrogen than amino acids, they are transported to other parts of the plant via xylem vessels.
In addition, along with the transpiration stream the nodules of some plants (e.g., soybean) export the fixed nitrogen as ureides.
These compounds also have a particularly high nitrogen-to-carbon ratio.
 Knowt
Knowt
